An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine
- PMID: 35956949
- PMCID: PMC9370529
- DOI: 10.3390/molecules27154999
An Exploration of the Effect of the Kleier Model and Carrier-Mediated Theory to Design Phloem-Mobile Pesticides Based on Researching the N-Alkylated Derivatives of Phenazine-1-Carboxylic Acid-Glycine
Abstract
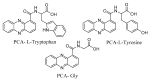
The Kleier model and Carrier-mediated theory are effective for molecularly designing pesticides with phloem mobility. However, the single Kleier model or Carrier-mediated theory cannot achieve a reliable explanation of the phloem mobility of all exogenous substances. A detailed investigation of the two models and the scope of their applications can provide a more accurate and highly efficient basis for the guidance of the design and development of phloem-mobile pesticides. In the present paper, a strategy using active ingredient-amino acid conjugates as mode compounds is developed based on Carrier-mediated theory. An N-alkylated amino acid is used to improve the pesticide’s physicochemical properties following the Kleier model, thus allowing the conjugates to fall on the predicted and more accessible transportation region of phloem. Moreover, the influence of this movement on phloem is inspected by the Kleier model and Carrier-mediated theory. To verify this strategy, a series of N-alkylated phenazine-1-carboxylic acid-glycine compounds (PCA-Gly) were designed and synthesized. The results related to the castor bean seeds (R. communis L.) indicated that all the target compounds (4a−4f) had phloem mobility. The capacity for phloem mobility shows that N-alkylated glycine containing small substituents can significantly improve PCA phloem mobility, such as 4c(i-C3H7-N) > 4a(CH3-N) ≈ 4b(C2H5-N) > 4d (t-C4H9-N) > PCA-Gly > 4e(C6H5-N) > 4f(CH2COOH-N), with an oil−water partition coefficient between 1.2~2.5. In particular, compounds 4a(CH3-N), 4b(C2H5-N), and 4c(i-C3H7-N) present better phloem mobility, with the average concentrations in phloem sap of 14.62 μΜ, 13.98 μΜ, and 17.63 μΜ in the first 5 h, which are 8 to 10 times higher than PCA-Gly (1.71 μΜ). The results reveal that the Kleier model and Carrier-mediated theory play a guiding role in the design of phloem-mobile pesticides. However, the single Kleier model or Carrier-mediated theory are not entirely accurate. Still, there is a synergism between Carrier-mediated theory and the Kleier model for promoting the phloem transport of exogenous compounds. Therefore, we suggest the introduction of endogenous plant compounds as a promoiety to improve the phloem mobility of pesticides through Carrier-mediated theory. It is necessary to consider the improvement of physicochemical properties according to the Kleier model, which can contribute to a scientific theory for developing phloem-mobile pesticides.
Keywords: Carrier-mediated theory; Kleier model; phenazine-1-carboxylic acid; phloem mobility; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Xu H.H., Zhang Z.X., Zha Y.G. The prospect of botanical pesticides in China. Pesticides. 2003;42:1–10.
-
- Nauen R., Reckmann U., Thomzik J., Thielert W. Biological profile of spirotetramat (Movento®)—A new two-way systemic (ambimobile) insecticide against sucking pest species. Bayer Crop Sci. J. 2008;2:245–278.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

