Lipase-Catalyzed Synthesis, Antioxidant Activity, Antimicrobial Properties and Molecular Docking Studies of Butyl Dihydrocaffeate
- PMID: 35956977
- PMCID: PMC9370587
- DOI: 10.3390/molecules27155024
Lipase-Catalyzed Synthesis, Antioxidant Activity, Antimicrobial Properties and Molecular Docking Studies of Butyl Dihydrocaffeate
Abstract
Green chemistry approaches, such as lipase-catalyzed esterification, are promising methods for obtaining valuable chemical compounds. In the case of the use of lipases, unlike in aqueous environments, the processes of the ester bond formations are encountered in organic solvents. The aim of the current research was to carry out the lipase-catalyzed synthesis of an ester of dihydrocaffeic acid. The synthesized compound was then evaluated for antioxidant and antimicrobial activities. However, the vast majority of its antioxidant activity was retained, which was demonstrated by means of DPPH· (2,2-diphenyl-1-picrylhydrazyl) and CUPRAC (cupric ion reducing antioxidant capacity) methods. Regarding its antimicrobial properties, the antifungal activity against Rhizopus oryzae is worth mentioning. The minimum inhibitory and fungicidal concentrations were 1 and 2 mM, respectively. The high antifungal activity prompted the use of molecular docking studies to verify potential protein targets for butyl ester of dihydrocaffeic ester. In the case of one fungal protein, namely 14-α sterol demethylase B, it was observed that the ester had comparable binding energy to the triazole medication, isavuconazole, but the interacted amino acid residues were different.
Keywords: Rhizopus oryzae; antifungal activity; butyl dihydrocaffeate; lipase-catalyzed synthesis; lipophilization; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
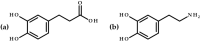
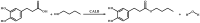



Similar articles
-
Lipase-catalyzed esterification of selected phenolic acids with linolenyl alcohols in organic solvent media.Appl Biochem Biotechnol. 2005 Oct;127(1):17-27. doi: 10.1385/abab:127:1:017. Appl Biochem Biotechnol. 2005. PMID: 16186620
-
Comparative study of the antioxidant activities of some lipase-catalyzed alkyl dihydrocaffeates synthesized in ionic liquid.Food Chem. 2017 Jun 1;224:365-371. doi: 10.1016/j.foodchem.2016.12.075. Epub 2016 Dec 22. Food Chem. 2017. PMID: 28159281
-
Production of butyl acetate ester by lipase from novel strain of Rhizopus oryzae.J Biosci Bioeng. 2007 Apr;103(4):368-72. doi: 10.1263/jbb.103.368. J Biosci Bioeng. 2007. PMID: 17502279
-
Recent advances in the enzymatic synthesis of lipophilic antioxidant and antimicrobial compounds.World J Microbiol Biotechnol. 2021 Dec 7;38(1):11. doi: 10.1007/s11274-021-03200-5. World J Microbiol Biotechnol. 2021. PMID: 34873650 Free PMC article. Review.
-
Advances in lipase-catalyzed esterification reactions.Biotechnol Adv. 2013 Dec;31(8):1846-59. doi: 10.1016/j.biotechadv.2013.08.006. Epub 2013 Aug 15. Biotechnol Adv. 2013. PMID: 23954307 Review.
Cited by
-
Dihydrocaffeic Acid-Is It the Less Known but Equally Valuable Phenolic Acid?Biomolecules. 2023 May 18;13(5):859. doi: 10.3390/biom13050859. Biomolecules. 2023. PMID: 37238728 Free PMC article. Review.
-
Profile and in silico analysis of metabolite compounds of the endophytic fungus Alternaria alternata K-10 from Drymoglossum piloselloides as antioxidants and antibacterials.Heliyon. 2024 Mar 11;10(6):e27978. doi: 10.1016/j.heliyon.2024.e27978. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524563 Free PMC article.
-
Optimizing lipase production by Bacillus subtilis on cheese whey and evaluating its antimicrobial, antibiofilm, anti virulence and biosafety properties.Sci Rep. 2025 Apr 1;15(1):11087. doi: 10.1038/s41598-025-92181-8. Sci Rep. 2025. PMID: 40169631 Free PMC article.
References
-
- Oliveira M.M., Ratti B.A., Dare R.G., Silva S.O., Truiti M.C.T., Ueda-Nakamura T., Auzely-Velty R., Nakamura C.V. Dihydrocaffeic Acid Prevents UVB-Induced Oxidative Stress Leading to the Inhibition of Apoptosis and MMP-1 Expression via p38 Signaling Pathway. Oxid. Med. Cell. Longev. 2019;2019:2419096. doi: 10.1155/2019/2419096. - DOI - PMC - PubMed
-
- Moon J.H., Terao J. Antioxidant Activity of Caffeic Acid and Dihydrocaffeic Acid in Lard and Human Low-Density Lipoprotein. J. Agric. Food Chem. 1998;46:5062–5065. doi: 10.1021/jf9805799. - DOI
-
- Baeza G., Sarria B., Mateos R., Bravo L. Dihydrocaffeic acid, a major microbial metabolite of chlorogenic acids, shows similar protective effect than a yerba mate phenolic extract against oxidative stress in HepG2 cells. Food Res. Int. 2018;87:25–33. doi: 10.1016/j.foodres.2016.06.011. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

