Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications
- PMID: 35957030
- PMCID: PMC9370329
- DOI: 10.3390/nano12152600
Control of the Drying Patterns for Complex Colloidal Solutions and Their Applications
Abstract
The uneven deposition at the edges of an evaporating droplet, termed the coffee-ring effect, has been extensively studied during the past few decades to better understand the underlying cause, namely the flow dynamics, and the subsequent patterns formed after drying. The non-uniform evaporation rate across the colloidal droplet hampers the formation of a uniform and homogeneous film in printed electronics, rechargeable batteries, etc., and often causes device failures. This review aims to highlight the diverse range of techniques used to alleviate the coffee-ring effect, from classic methods such as adding chemical additives, applying external sources, and manipulating geometrical configurations to recently developed advancements, specifically using bubbles, humidity, confined systems, etc., which do not involve modification of surface, particle or liquid properties. Each of these methodologies mitigates the edge deposition via multi-body interactions, for example, particle-liquid, particle-particle, particle-solid interfaces and particle-flow interactions. The mechanisms behind each of these approaches help to find methods to inhibit the non-uniform film formation, and the corresponding applications have been discussed together with a critical comparison in detail. This review could pave the way for developing inks and processes to apply in functional coatings and printed electronic devices with improved efficiency and device yield.
Keywords: coffee-ring effect; deposition patterns; evaporation; interfacial flow.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





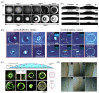


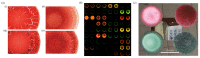
References
-
- Liu S., Zhang X., Yin M., Feng H., Zhang J., Zhang L., Xie W. Coffee-ring-free ultrasonic spray coating single-emission layers for white organic light-emitting devices and their energy-transfer mechanism. ACS Appl. Energy Mater. 2017;1:103–112. doi: 10.1021/acsaem.7b00011. - DOI
-
- Deng Y., Wang Q., Yuan Y., Huang J. Vividly colorful hybrid perovskite solar cells by doctor-blade coating with perovskite photonic nanostructures. Mater. Horiz. 2015;2:578–583. doi: 10.1039/C5MH00126A. - DOI
-
- Jiang C., Ng S.M., Leung C.W., Pong P.W. Magnetically assembled iron oxide nanoparticle coatings and their integration with pseudo-spin-valve thin films. J. Mater. Chem. C. 2017;5:252–263. doi: 10.1039/C6TC03918A. - DOI
-
- Tiara A., Moon H., Cho G., Lee J. Fully roll-to-roll gravure printed electronics: Challenges and the way to integrating logic gates. Jpn. J. Appl. Phys. 2022;61:SE0802. doi: 10.35848/1347-4065/ac575e. - DOI
-
- He P., Derby B. Controlling coffee ring formation during drying of inkjet printed 2D inks. Adv. Mater. Interfaces. 2017;4:1700944. doi: 10.1002/admi.201700944. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

