On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial
- PMID: 35957039
- PMCID: PMC9370519
- DOI: 10.3390/nano12152608
On the Morphology of Nanostructured TiO2 for Energy Applications: The Shape of the Ubiquitous Nanomaterial
Abstract
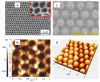
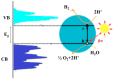
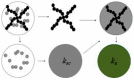
Nanostructured titania is one of the most commonly encountered constituents of nanotechnology devices for use in energy-related applications, due to its intrinsic functional properties as a semiconductor and to other favorable characteristics such as ease of production, low toxicity and chemical stability, among others. Notwithstanding this diffusion, the quest for improved understanding of the physical and chemical mechanisms governing the material properties and thus its performance in devices is still active, as testified by the large number of dedicated papers that continue to be published. In this framework, we consider and analyze here the effects of the material morphology and structure in determining the energy transport phenomena as cross-cutting properties in some of the most important nanophase titania applications in the energy field, namely photovoltaic conversion, hydrogen generation by photoelectrochemical water splitting and thermal management by nanofluids. For these applications, charge transport, light transport (or propagation) and thermal transport are limiting factors for the attainable performances, whose dependence on the material structural properties is reviewed here on its own. This work aims to fill the gap existing among the many studies dealing with the separate applications in the hope of stimulating novel cross-fertilization approaches in this research field.
Keywords: dye-sensitized solar cells; nanofluids; nanophase TiO2; perovskite solar cells; photoelectrochemical water splitting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Solution-Processed Anatase Titania Nanowires: From Hyperbranched Design to Optoelectronic Applications.Acc Chem Res. 2019 Mar 19;52(3):633-644. doi: 10.1021/acs.accounts.8b00476. Epub 2019 Jan 22. Acc Chem Res. 2019. PMID: 30668116
-
Understanding Charge Transport in Carbon Nitride for Enhanced Photocatalytic Solar Fuel Production.Acc Chem Res. 2019 Jan 15;52(1):248-257. doi: 10.1021/acs.accounts.8b00542. Epub 2018 Dec 31. Acc Chem Res. 2019. PMID: 30596234
-
Perovskite solar cell for photocatalytic water splitting with a TiO2/Co-doped hematite electron transport bilayer.RSC Adv. 2018 Jan 31;8(10):5388-5394. doi: 10.1039/c7ra11996h. eCollection 2018 Jan 29. RSC Adv. 2018. PMID: 35542422 Free PMC article.
-
Sustainable hydrogen production from water using tandem dye-sensitized photoelectrochemical cells.Nano Converg. 2021 Mar 2;8(1):7. doi: 10.1186/s40580-021-00257-8. Nano Converg. 2021. PMID: 33650039 Free PMC article. Review.
-
Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting.Small. 2021 Mar;17(9):e1903378. doi: 10.1002/smll.201903378. Epub 2019 Oct 28. Small. 2021. PMID: 31657147 Review.
Cited by
-
Novel Sol-Gel Synthesis of TiO2 Spherical Porous Nanoparticles Assemblies with Photocatalytic Activity.Nanomaterials (Basel). 2023 Jun 25;13(13):1928. doi: 10.3390/nano13131928. Nanomaterials (Basel). 2023. PMID: 37446444 Free PMC article.
References
-
- Akakuru O.U., Iqbal Z.M., Wu A. TiO2 Nanoparticles. John Wiley & Sons, Ltd.; New York, NY, USA: 2020. TiO2 Nanoparticles: Properties and applications; pp. 1–66. - DOI
-
- Lan Y., Cook L., Lisfi A. Titanium Dioxide Nanoparticles: Characterizations, Properties and Syntheses. NOVA Science Publisher; Hauppauge, NY, USA: 2017. New Applications of TiO2 Nanoparticles in Renewable Energy; p. 241.
-
- Ijaz M., Zafar M. Titanium Dioxide Nanostructures as Efficient Photocatalyst: Progress, Challenges and Perspective. Int. J. Energy Res. 2021;45:3569–3589. doi: 10.1002/er.6079. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

