Synthesis of Uniform Size Rutile TiO2 Microrods by Simple Molten-Salt Method and Its Photoluminescence Activity
- PMID: 35957057
- PMCID: PMC9370513
- DOI: 10.3390/nano12152626
Synthesis of Uniform Size Rutile TiO2 Microrods by Simple Molten-Salt Method and Its Photoluminescence Activity
Abstract
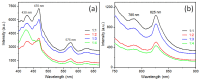
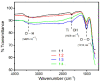
Uniform-size rutile TiO2 microrods were synthesized by simple molten-salt method with sodium chloride as reacting medium and different kinds of sodium phosphate salts as growth control additives to control the one-dimensional (1-D) crystal growth of particles. The effect of rutile and anatase ratios as a precursor was monitored for rod growth formation. Apart from uniform rod growth study, optical properties of rutile microrods were observed by UV-visible and photoluminescence (PL) spectroscopy. TiO2 materials with anatase and rutile phase show PL emission due to self-trapped exciton. It has been observed that synthesized rutile TiO2 rods show various PL emission peaks in the range of 400 to 900 nm for 355 nm excitation wavelengths. All PL emission appeared due to the oxygen vacancy present inside rutile TiO2 rods. The observed PL near the IR range (785 and 825 nm) was due to the formation of a self-trapped hole near to the surface of (110) which is the preferred orientation plane of synthesized rutile TiO2 microrods.
Keywords: TiO2 microrod; anatase; molten-salt method; photoluminescence; rutile.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Photoluminescence of anatase and rutile TiO2 particles.J Phys Chem B. 2006 Dec 21;110(50):25366-70. doi: 10.1021/jp064454f. J Phys Chem B. 2006. PMID: 17165983
-
Oxygen induced enhancement of NIR emission in brookite TiO2 powders: comparison with rutile and anatase TiO2 powders.Phys Chem Chem Phys. 2018 Jan 31;20(5):3241-3248. doi: 10.1039/c7cp06975h. Phys Chem Chem Phys. 2018. PMID: 29105714
-
Quantum dynamics origin of high photocatalytic activity of mixed-phase anatase/rutile TiO2.J Chem Phys. 2020 Jul 28;153(4):044706. doi: 10.1063/5.0014179. J Chem Phys. 2020. PMID: 32752673
-
TiO2-Based Nanomaterials for Gas Sensing-Influence of Anatase and Rutile Contributions.Nanoscale Res Lett. 2017 Dec;12(1):89. doi: 10.1186/s11671-017-1875-5. Epub 2017 Feb 6. Nanoscale Res Lett. 2017. PMID: 28168614 Free PMC article.
-
UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk.J Phys Chem B. 2006 Jan 19;110(2):927-35. doi: 10.1021/jp0552473. J Phys Chem B. 2006. PMID: 16471625
Cited by
-
Special Issue "Synthesis of TiO2 Nanoparticles and Their Catalytic Activity".Nanomaterials (Basel). 2023 Sep 12;13(18):2544. doi: 10.3390/nano13182544. Nanomaterials (Basel). 2023. PMID: 37764573 Free PMC article.
-
Electrochemical Determination of Cyclobenzaprine Hydrochloride Muscle Relaxant Using Novel S-GCN/TiO2-Based Carbon Electrode.ACS Omega. 2024 Jul 12;9(29):31657-31668. doi: 10.1021/acsomega.4c02158. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072069 Free PMC article.
-
Pure Epigallocatechin-3-gallate-Assisted Green Synthesis of Highly Stable Titanium Dioxide Nanoparticles.Materials (Basel). 2024 Jan 5;17(2):275. doi: 10.3390/ma17020275. Materials (Basel). 2024. PMID: 38255442 Free PMC article.
References
-
- Wedd M., Ward-Smith S., Rawle A. Encyclopedia of Analytical Science. Elsevier; Amsterdam, The Netherlands: 2019. Particle Size Analysis; pp. 144–157.
-
- Trogadas P., Fuller T.F. The Effect of Uniform Particle Size Distribution on Pt Stability. ECS Trans. 2011;41:761–773. doi: 10.1149/1.3635610. - DOI
-
- Nan J., Huang C., Tian L., Shen C. Effects of micro-emulsion method on microwave dielectric properties of 0.9Al2O3-0.1TiO2 ceramics. Mater. Lett. 2019;249:132–135. doi: 10.1016/j.matlet.2019.04.080. - DOI
-
- Shivaraj B., Prabhakara M.C., Naik H.S.B., Naik E.I., Viswanath R., Shashank M., Swamy B.E.K. Optical, bio-sensing, and antibacterial studies on Ni-doped ZnO nanorods, fabricated by chemical co-precipitation method. Inorg. Chem. Commun. 2021;134:109049. doi: 10.1016/j.inoche.2021.109049. - DOI
-
- Lee B.T., Han J.K., Gain A.K., Lee K.H., Saito F. TEM microstructure characterization of nano TiO2 coated on nano ZrO2 powders and their photocatalytic activity. Mater. Lett. 2006;60:2101–2104. doi: 10.1016/j.matlet.2005.12.102. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

