Efficient Electrocatalyst Nanoparticles from Upcycled Class II Capacitors
- PMID: 35957128
- PMCID: PMC9370706
- DOI: 10.3390/nano12152697
Efficient Electrocatalyst Nanoparticles from Upcycled Class II Capacitors
Abstract
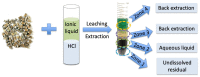
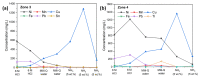
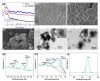
To move away from fossil fuels, the electrochemical reaction plays a critical role in renewable energy sources and devices. The anodic oxygen evolution reaction (OER) is always coupled with these reactions in devices but suffers from large energy barriers. Thus, it is important for developing efficient OER catalysts with low overpotential. On the other hand, there are large amounts of metals in electronic waste (E-waste), especially various transition metals that are promising alternatives for catalyzing OER. Hence, this work, which focuses on upcycling Class II BaTiO3 Multilayer Ceramic Capacitors, of which two trillion were produced in 2011 alone. We achieved this by first using a green solvent extraction method that combined the ionic liquid Aliquat® 336 and hydrochloride acid to recover a mixed solution of Ni, Fe and Cu cations, and then using such a solution to synthesize high potential catalysts NiFe hydroxide and NiCu hydroxide for OER. NiFe-hydroxide has been demonstrated to have faster OER kinetics than the NiCu-hydroxide and commercial c-RuO2. In addition, it showed promising results after the chronopotentiometry tests that outperform c-RuO2.
Keywords: ceramic capacitor; circular economy; electrocatalysis; electronic waste; ionic liquid; layered double hydroxide; liquid-liquid extraction; nanoparticle; nickel; re-use; recycling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of Ketjenblack Decorated Pillared Ni(Fe) Metal-Organic Frameworks as Precursor Electrocatalysts for Enhancing the Oxygen Evolution Reaction.Molecules. 2023 May 31;28(11):4464. doi: 10.3390/molecules28114464. Molecules. 2023. PMID: 37298940 Free PMC article.
-
Boosting the inherent activity of NiFe layered double hydroxide via erbium incorporation for water oxidation.Front Chem. 2023 Aug 24;11:1261332. doi: 10.3389/fchem.2023.1261332. eCollection 2023. Front Chem. 2023. PMID: 37693173 Free PMC article.
-
Magnetic Heating Amorphous NiFe Hydroxide Nanosheets Encapsulated Ni Nanoparticles@Wood Carbon to Boost Oxygen Evolution Reaction Activity.Small. 2023 Jun;19(26):e2206798. doi: 10.1002/smll.202206798. Epub 2023 Apr 3. Small. 2023. PMID: 37010010
-
Facile formation of Zn-incorporated NiFe layered double hydroxide as highly-efficient oxygen evolution catalyst.J Colloid Interface Sci. 2023 Oct;647:65-72. doi: 10.1016/j.jcis.2023.05.123. Epub 2023 May 22. J Colloid Interface Sci. 2023. PMID: 37244177
-
Recent Progress on Nickel-Based Oxide/(Oxy)Hydroxide Electrocatalysts for the Oxygen Evolution Reaction.Chemistry. 2019 Jan 14;25(3):703-713. doi: 10.1002/chem.201802068. Epub 2018 Nov 9. Chemistry. 2019. PMID: 30024645 Review.
Cited by
-
Drivers and Pathways for the Recovery of Critical Metals from Waste-Printed Circuit Boards.Adv Sci (Weinh). 2024 Aug;11(30):e2309635. doi: 10.1002/advs.202309635. Epub 2024 Jun 5. Adv Sci (Weinh). 2024. PMID: 38837685 Free PMC article. Review.
-
Waste-Derived Catalysts for Water Electrolysis: Circular Economy-Driven Sustainable Green Hydrogen Energy.Nanomicro Lett. 2022 Dec 1;15(1):4. doi: 10.1007/s40820-022-00974-7. Nanomicro Lett. 2022. PMID: 36454315 Free PMC article. Review.
-
Random Access Memory (RAM) Contacts Waste Catalyzes Organic Reactions.Glob Chall. 2025 May 8;9(6):2500069. doi: 10.1002/gch2.202500069. eCollection 2025 Jun. Glob Chall. 2025. PMID: 40510649 Free PMC article.
References
-
- Baldé K., Wang F., Kuehr R., Huisman J. The Global E-Waste Monitor—2014. United Nations University, IAS—SCYCLE; Bonn, Germany: 2015.
-
- Rai V., Liu D., Xia D., Gabriel J.C.P. Electrochemical Approaches for the Recovery of Metals from Electronic Waste, a Critical Review. Recycling. 2021;6:53. doi: 10.3390/recycling6030053. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

