Planar Junctionless Field-Effect Transistor for Detecting Biomolecular Interactions
- PMID: 35957340
- PMCID: PMC9371156
- DOI: 10.3390/s22155783
Planar Junctionless Field-Effect Transistor for Detecting Biomolecular Interactions
Abstract
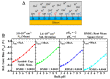
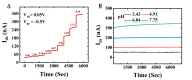
Label-free field-effect transistor-based immunosensors are promising candidates for proteomics and peptidomics-based diagnostics and therapeutics due to their high multiplexing capability, fast response time, and ability to increase the sensor sensitivity due to the short length of peptides. In this work, planar junctionless field-effect transistor sensors (FETs) were fabricated and characterized for pH sensing. The device with SiO2 gate oxide has shown voltage sensitivity of 41.8 ± 1.4, 39.9 ± 1.4, 39.0 ± 1.1, and 37.6 ± 1.0 mV/pH for constant drain currents of 5, 10, 20, and 50 nA, respectively, with a drain to source voltage of 0.05 V. The drift analysis shows a stability over time of -18 nA/h (pH 7.75), -3.5 nA/h (pH 6.84), -0.5 nA/h (pH 4.91), 0.5 nA/h (pH 3.43), corresponding to a pH drift of -0.45, -0.09, -0.01, and 0.01 per h. Theoretical modeling and simulation resulted in a mean value of the surface states of 3.8 × 1015/cm2 with a standard deviation of 3.6 × 1015/cm2. We have experimentally verified the number of surface sites due to APTES, peptide, and protein immobilization, which is in line with the theoretical calculations for FETs to be used for detecting peptide-protein interactions for future applications.
Keywords: diagnostics; pH sensor; peptide-protein interaction; peptidomics; planar junctionless FETs; proteomics; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Enhanced sensitivity of double gate junctionless transistor architecture for biosensing applications.Nanotechnology. 2015 Apr 10;26(14):145201. doi: 10.1088/0957-4484/26/14/145201. Epub 2015 Mar 16. Nanotechnology. 2015. PMID: 25771821
-
Silicon Nanowire Field Effect Transistor Sensors with Minimal Sensor-to-Sensor Variations and Enhanced Sensing Characteristics.ACS Nano. 2018 Jul 24;12(7):6577-6587. doi: 10.1021/acsnano.8b01339. Epub 2018 Jul 5. ACS Nano. 2018. PMID: 29932634
-
Improved sensing characteristics of dual-gate transistor sensor using silicon nanowire arrays defined by nanoimprint lithography.Sci Technol Adv Mater. 2017 Jan 6;18(1):17-25. doi: 10.1080/14686996.2016.1253409. eCollection 2017. Sci Technol Adv Mater. 2017. PMID: 28179955 Free PMC article.
-
A review on nanomaterial-based field effect transistor technology for biomarker detection.Mikrochim Acta. 2019 Nov 1;186(11):739. doi: 10.1007/s00604-019-3850-6. Mikrochim Acta. 2019. PMID: 31677098 Review.
-
Dual-gate thin-film transistors, integrated circuits and sensors.Adv Mater. 2011 Aug 2;23(29):3231-42. doi: 10.1002/adma.201101493. Epub 2011 Jun 14. Adv Mater. 2011. PMID: 21671446 Review.
Cited by
-
Acid-Modulated Peptide Synthesis for Application on Oxide Biosensor Interfaces.Nanomaterials (Basel). 2023 Dec 6;13(24):3092. doi: 10.3390/nano13243092. Nanomaterials (Basel). 2023. PMID: 38132988 Free PMC article.
-
A Novel Bulk Planar Junctionless Field-Effect Transistor for High-Performance Biosensing.Biosensors (Basel). 2025 Feb 22;15(3):135. doi: 10.3390/bios15030135. Biosensors (Basel). 2025. PMID: 40136932 Free PMC article.
-
Design of a novel high-sensitive SOI-Junctionless BioFET overcoming sensitivity degradation problems.Sci Rep. 2024 Aug 8;14(1):18395. doi: 10.1038/s41598-024-69383-7. Sci Rep. 2024. PMID: 39117858 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

