Detection of Shiga Toxin-Producing Escherichia coli (STEC) in the Endocervix of Asymptomatic Pregnant Women. Can STEC Be a Risk Factor for Adverse Pregnancy Outcomes?
- PMID: 35957815
- PMCID: PMC9358589
- DOI: 10.3389/fendo.2022.945736
Detection of Shiga Toxin-Producing Escherichia coli (STEC) in the Endocervix of Asymptomatic Pregnant Women. Can STEC Be a Risk Factor for Adverse Pregnancy Outcomes?
Abstract
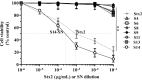
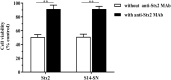
The presence of Escherichia coli in the vaginal microbiome has been associated with pregnancy complications. In previous works, we demonstrated that Shiga toxin-producing Escherichia coli (STEC) can produce abortion and premature delivery in rats and that Shiga toxin type 2 (Stx2) can impair human trophoblast cell lines. The hypothesis of this work was that STEC may colonize the lower female reproductive tract and be responsible for adverse pregnancy outcomes. Thus, the aim of this work was to evaluate the presence and prevalence of virulence factor genes from STEC in the endocervix of asymptomatic pregnant women. For that purpose, endocervical swabs were collected from pregnant women during their prenatal examination. Swab samples were enriched in a differential medium to select Enterobacteria. Then, positive samples were analyzed by PCR to detect genes characteristic of Escherichia sp. (such as uidA and yaiO), genes specific for portions of the rfb (O-antigen-encoding) regions of STEC O157 (rfbO157), and STEC virulence factor genes (such as stx1, stx2, eae, lpfAO113, hcpA, iha, sab, subAB). The cytotoxic effects of stx2-positive supernatants from E. coli recovered from the endocervix were evaluated in Vero cells. Our results showed that 11.7% of the endocervical samples were positive for E. coli. Additionally, we found samples positive for stx2 and other virulence factors for STEC. The bacterial supernatant from an isolate identified as E. coli O113:NT, carrying the stx2 gene, exhibited cytotoxic activity in Vero, Swan 71 and Hela cells. Our results open a new perspective regarding the presence of STEC during pregnancy.
Keywords: Endocervical microbiota; STEC; Stx2; pregnancy; virulence factors.
Copyright © 2022 Scalise, Garimano, Sanz, Padola, Leonino, Pereyra, Casale, Amaral, Sacerdoti and Ibarra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

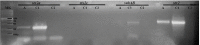

Similar articles
-
Virulent shiga toxin-producing Escherichia coli (STEC) O157:H7 ST11 isolated from ground beef in Brazil.Braz J Microbiol. 2024 Dec;55(4):3513-3520. doi: 10.1007/s42770-024-01468-x. Epub 2024 Jul 31. Braz J Microbiol. 2024. PMID: 39083224 Free PMC article.
-
Virulence genes, Shiga toxin subtypes, major O-serogroups, and phylogenetic background of Shiga toxin-producing Escherichia coli strains isolated from cattle in Iran.Microb Pathog. 2017 Aug;109:274-279. doi: 10.1016/j.micpath.2017.05.041. Epub 2017 May 31. Microb Pathog. 2017. PMID: 28578089
-
Shiga toxin-producing Escherichia coli in drinking water supplies of north Paraná State, Brazil.J Appl Microbiol. 2013 Apr;114(4):1230-9. doi: 10.1111/jam.12113. Epub 2013 Jan 14. J Appl Microbiol. 2013. PMID: 23279284
-
Regional differences in presence of Shiga toxin-producing Escherichia coli virulence-associated genes in the environment in the North West and East Anglian regions of England.Lett Appl Microbiol. 2020 Aug;71(2):179-186. doi: 10.1111/lam.13303. Epub 2020 May 15. Lett Appl Microbiol. 2020. PMID: 32333799
-
Shiga Toxin-Producing Escherichia coli Infections during Pregnancy.Microorganisms. 2018 Oct 23;6(4):111. doi: 10.3390/microorganisms6040111. Microorganisms. 2018. PMID: 30360505 Free PMC article. Review.
Cited by
-
Rapid Bacteria Detection from Patients' Blood Bypassing Classical Bacterial Culturing.Biosensors (Basel). 2022 Nov 9;12(11):994. doi: 10.3390/bios12110994. Biosensors (Basel). 2022. PMID: 36354504 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

