Alpha-cell paracrine signaling in the regulation of beta-cell insulin secretion
- PMID: 35957816
- PMCID: PMC9360487
- DOI: 10.3389/fendo.2022.934775
Alpha-cell paracrine signaling in the regulation of beta-cell insulin secretion
Abstract
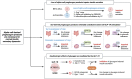
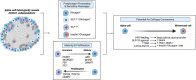
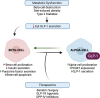
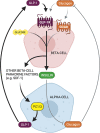
As an incretin hormone, glucagon-like peptide 1 (GLP-1) lowers blood glucose levels by enhancing glucose-stimulated insulin secretion from pancreatic beta-cells. Therapies targeting the GLP-1 receptor (GLP-1R) use the classical incretin model as a physiological framework in which GLP-1 secreted from enteroendocrine L-cells acts on the beta-cell GLP-1R. However, this model has come into question, as evidence demonstrating local, intra-islet GLP-1 production has advanced the competing hypothesis that the incretin activity of GLP-1 may reflect paracrine signaling of GLP-1 from alpha-cells on GLP-1Rs on beta-cells. Additionally, recent studies suggest that alpha-cell-derived glucagon can serve as an additional, albeit less potent, ligand for the beta-cell GLP-1R, thereby expanding the role of alpha-cells beyond that of a counterregulatory cell type. Efforts to understand the role of the alpha-cell in the regulation of islet function have revealed both transcriptional and functional heterogeneity within the alpha-cell population. Further analysis of this heterogeneity suggests that functionally distinct alpha-cell subpopulations display alterations in islet hormone profile. Thus, the role of the alpha-cell in glucose homeostasis has evolved in recent years, such that alpha-cell to beta-cell communication now presents a critical axis regulating the functional capacity of beta-cells. Herein, we describe and integrate recent advances in our understanding of the impact of alpha-cell paracrine signaling on insulin secretory dynamics and how this intra-islet crosstalk more broadly contributes to whole-body glucose regulation in health and under metabolic stress. Moreover, we explore how these conceptual changes in our understanding of intra-islet GLP-1 biology may impact our understanding of the mechanisms of incretin-based therapeutics.
Keywords: GLP-1; alpha-cell; beta-cell; glucagon; insulin; paracrine signaling.
Copyright © 2022 Holter, Saikia and Cummings.
Conflict of interest statement
The authors declare that this review was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Intra-islet glucagon confers β-cell glucose competence for first-phase insulin secretion and favors GLP-1R stimulation by exogenous glucagon.J Biol Chem. 2022 Feb;298(2):101484. doi: 10.1016/j.jbc.2021.101484. Epub 2021 Dec 9. J Biol Chem. 2022. PMID: 34896391 Free PMC article.
-
Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells.Metabolism. 2014 Jan;63(1):9-19. doi: 10.1016/j.metabol.2013.09.010. Epub 2013 Oct 17. Metabolism. 2014. PMID: 24140094 Review.
-
Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis.J Biol Chem. 2016 Mar 25;291(13):6626-40. doi: 10.1074/jbc.M115.699504. Epub 2016 Jan 12. J Biol Chem. 2016. PMID: 26757816 Free PMC article.
-
Is GLP-1 a hormone: Whether and When?J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):50-5. doi: 10.1111/jdi.12466. Epub 2016 Mar 14. J Diabetes Investig. 2016. PMID: 27186356 Free PMC article. Review.
-
SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells.Mol Cell Biol. 2013 Jul;33(13):2527-34. doi: 10.1128/MCB.00285-13. Epub 2013 Apr 29. Mol Cell Biol. 2013. PMID: 23629625 Free PMC article.
Cited by
-
Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges.Drugs. 2024 Feb;84(2):127-148. doi: 10.1007/s40265-023-01982-6. Epub 2023 Dec 21. Drugs. 2024. PMID: 38127286 Review.
-
A metabolic dysfunction-associated steatotic liver acinus biomimetic induces pancreatic islet dysfunction in a coupled microphysiology system.Commun Biol. 2024 Oct 14;7(1):1317. doi: 10.1038/s42003-024-07006-7. Commun Biol. 2024. PMID: 39397070 Free PMC article.
-
Developmental programming: An exploratory analysis of pancreatic islet compromise in female sheep resulting from gestational BPA exposure.Mol Cell Endocrinol. 2024 Jul 1;588:112202. doi: 10.1016/j.mce.2024.112202. Epub 2024 Mar 27. Mol Cell Endocrinol. 2024. PMID: 38552943 Free PMC article.
-
GPCR-mediated effects of fatty acids and bile acids on glucose homeostasis.Front Endocrinol (Lausanne). 2023 Jul 7;14:1206063. doi: 10.3389/fendo.2023.1206063. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37484954 Free PMC article. Review.
-
Ginger extract promotes pancreatic islets regeneration in streptozotocin-induced diabetic rats.Biosci Rep. 2025 Mar 13;45(3):BSR20241510. doi: 10.1042/BSR20241510. Biosci Rep. 2025. PMID: 40014427 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

