Paeoniflorin Inhibits EMT and Angiogenesis in Human Glioblastoma via K63-Linked C-Met Polyubiquitination-Dependent Autophagic Degradation
- PMID: 35957872
- PMCID: PMC9360619
- DOI: 10.3389/fonc.2022.785345
Paeoniflorin Inhibits EMT and Angiogenesis in Human Glioblastoma via K63-Linked C-Met Polyubiquitination-Dependent Autophagic Degradation
Abstract
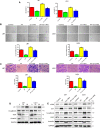
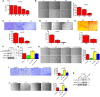
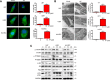
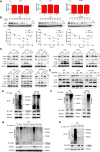
Epithelial-to-mesenchymal transition (EMT) and angiogenesis have emerged as two pivotal events in cancer progression. Paeoniflorin has been widely studied in experimental models and clinical trials for cancer treatment because of its anti-cancer property. However, the underlying mechanisms of paeoniflorin in EMT and angiogenesis in glioblastoma was not fully elucidated. The present study aimed to investigate whether paeoniflorin inhibits EMT and angiogenesis, which involving c-Met suppression, while exploring the potential ways of c-Met degradation. In our study, we found that paeoniflorin inhibited EMT via downregulating c-Met signaling in glioblastoma cells. Furthermore, overexpressing c-Met in glioblastoma cells abolished the effects of paeoniflorin on EMT. Moreover, paeoniflorin showed anti-angiogenic effects by suppressing cell proliferation, migration, invasion and tube formation through downregulating c-Met in human umbilical vein endothelial cells (HUVECs). And c-Met overexpression in HUVECs offset the effects of paeoniflorin on angiogenesis. Additionally, paeoniflorin induced autophagy activation involving mTOR/P70S6K/S6 signaling and promoted c-Met autophagic degradation, a process dependent on K63-linked c-Met polyubiquitination. Finally, paeoniflorin suppressed mesenchymal makers (snail, vimentin, N-cadherin) and inhibited angiogenesis via the identical mechanism in an orthotopic xenograft mouse model. The in vitro and in vivo experiments showed that paeoniflorin treatment inhibited EMT, angiogenesis and activated autophagy. What's more, for the first time, we identified c-Met may be a potential target of paeoniflorin and demonstrated paeoniflorin downregulated c-Met via K63-linked c-Met polyubiquitination-dependent autophagic degradation. Collectively, these findings indicated that paeoniflorin inhibits EMT and angiogenesis via K63-linked c-Met polyubiquitination-dependent autophagic degradation in human glioblastoma.
Keywords: EMT; angiogenesis; autophagy; c-Met; glioblastoma; paeoniflorin; polyubiquitination.
Copyright © 2022 Liu, Wang, Chen, Liu, Yu, Zhang, Chen, Xu, Wang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Raddeanin A inhibited epithelial-mesenchymal transition (EMT) and angiogenesis in glioblastoma by downregulating β-catenin expression.Int J Med Sci. 2021 Feb 4;18(7):1609-1617. doi: 10.7150/ijms.52206. eCollection 2021. Int J Med Sci. 2021. PMID: 33746577 Free PMC article.
-
Paeoniflorin Inhibits Migration and Invasion of Human Glioblastoma Cells via Suppression Transforming Growth Factor β-Induced Epithelial-Mesenchymal Transition.Neurochem Res. 2018 Mar;43(3):760-774. doi: 10.1007/s11064-018-2478-y. Epub 2018 Feb 8. Neurochem Res. 2018. PMID: 29423667 Free PMC article.
-
Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer.Mol Ther Oncolytics. 2016 Aug 3;3:16018. doi: 10.1038/mto.2016.18. eCollection 2016. Mol Ther Oncolytics. 2016. PMID: 27525306 Free PMC article.
-
Verbascoside suppresses the migration and invasion of human glioblastoma cells via targeting c-Met-mediated epithelial-mesenchymal transition.Biochem Biophys Res Commun. 2019 Jul 5;514(4):1270-1277. doi: 10.1016/j.bbrc.2019.05.096. Epub 2019 May 18. Biochem Biophys Res Commun. 2019. PMID: 31113618
-
Multifaceted WNT Signaling at the Crossroads Between Epithelial-Mesenchymal Transition and Autophagy in Glioblastoma.Front Oncol. 2020 Nov 12;10:597743. doi: 10.3389/fonc.2020.597743. eCollection 2020. Front Oncol. 2020. PMID: 33312955 Free PMC article. Review.
Cited by
-
Paeoniflorin Attenuates Limb Ischemia by Promoting Angiogenesis Through ERα/ROCK-2 Pathway.Pharmaceuticals (Basel). 2025 Feb 19;18(2):272. doi: 10.3390/ph18020272. Pharmaceuticals (Basel). 2025. PMID: 40006085 Free PMC article.
-
Paeoniflorin inhibits colorectal cancer cell stemness through the miR-3194-5p/catenin beta-interacting protein 1 axis.Kaohsiung J Med Sci. 2023 Oct;39(10):1011-1021. doi: 10.1002/kjm2.12736. Epub 2023 Aug 2. Kaohsiung J Med Sci. 2023. PMID: 37530655 Free PMC article.
-
Cellular senescence and metabolic reprogramming: Unraveling the intricate crosstalk in the immunosuppressive tumor microenvironment.Cancer Commun (Lond). 2024 Sep;44(9):929-966. doi: 10.1002/cac2.12591. Epub 2024 Jul 12. Cancer Commun (Lond). 2024. PMID: 38997794 Free PMC article. Review.
-
Deciphering the mechanism of PSORI-CM02 in suppressing keratinocyte proliferation through the mTOR/HK2/glycolysis axis.Front Pharmacol. 2023 Apr 7;14:1152347. doi: 10.3389/fphar.2023.1152347. eCollection 2023. Front Pharmacol. 2023. PMID: 37089953 Free PMC article.
-
Integrating Network Pharmacology and Experimental Verification to Explore the Pharmacological Mechanisms of Radix Paeoniae Rubra Against Glioma.Appl Biochem Biotechnol. 2024 Sep;196(9):6424-6441. doi: 10.1007/s12010-024-04887-6. Epub 2024 Feb 21. Appl Biochem Biotechnol. 2024. PMID: 38381309
References
-
- Khan MK, Hunter GK, Vogelbaum M, Suh JH, Chao ST. Evidence-Based Adjuvant Therapy for Gliomas: Current Concepts and Newer Developments. Indian J Cancer (2009) 46(2):96–107. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

