RET signaling pathway and RET inhibitors in human cancer
- PMID: 35957881
- PMCID: PMC9359433
- DOI: 10.3389/fonc.2022.932353
RET signaling pathway and RET inhibitors in human cancer
Abstract
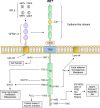
Rearranged during transfection (RET) receptor tyrosine kinase was first identified over thirty years ago as a novel transforming gene. Since its discovery and subsequent pathway characterization, RET alterations have been identified in numerous cancer types and are most prevalent in thyroid carcinomas and non-small cell lung cancer (NSCLC). In other tumor types such as breast cancer and salivary gland carcinomas, RET alterations can be found at lower frequencies. Aberrant RET activity is associated with poor prognosis of thyroid and lung carcinoma patients, and is strongly correlated with increased risk of distant metastases. RET aberrations encompass a variety of genomic or proteomic alterations, most of which confer constitutive activation of RET. Activating RET alterations, such as point mutations or gene fusions, enhance activity of signaling pathways downstream of RET, namely PI3K/AKT, RAS/RAF, MAPK, and PLCγ pathways, to promote cell proliferation, growth, and survival. Given the important role that mutant RET plays in metastatic cancers, significant efforts have been made in developing inhibitors against RET kinase activity. These efforts have led to FDA approval of Selpercatinib and Pralsetinib for NSCLC, as well as, additional selective RET inhibitors in preclinical and clinical testing. This review covers the current biological understanding of RET signaling, the impact of RET hyperactivity on tumor progression in multiple tumor types, and RET inhibitors with promising preclinical and clinical efficacy.
Keywords: RET; cancer; lung cancer; therapeutics; thyroid cancer.
Copyright © 2022 Regua, Najjar and Lo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Precision oncology with selective RET inhibitor selpercatinib in RET-rearranged cancers.Ther Adv Med Oncol. 2023 Jun 21;15:17588359231177015. doi: 10.1177/17588359231177015. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37360768 Free PMC article. Review.
-
An integrative pan cancer analysis of RET aberrations and their potential clinical implications.Sci Rep. 2022 Aug 17;12(1):13913. doi: 10.1038/s41598-022-17791-y. Sci Rep. 2022. PMID: 35978072 Free PMC article.
-
Predictive molecular pathology in metastatic thyroid cancer: the role of RET fusions.Expert Rev Endocrinol Metab. 2022 Mar;17(2):167-178. doi: 10.1080/17446651.2022.2060819. Epub 2022 Apr 11. Expert Rev Endocrinol Metab. 2022. PMID: 35404189 Review.
-
Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer.Ann Oncol. 2020 Dec;31(12):1725-1733. doi: 10.1016/j.annonc.2020.09.015. Epub 2020 Sep 29. Ann Oncol. 2020. PMID: 33007380 Free PMC article.
-
An updated patent review of rearranged during transfection (RET) kinase inhibitors (2016-present).Expert Opin Ther Pat. 2022 Oct;32(10):1067-1077. doi: 10.1080/13543776.2022.2132851. Epub 2022 Oct 18. Expert Opin Ther Pat. 2022. PMID: 36198171 Review.
Cited by
-
RET rearrangement-positive pancreatic cancer has remarkable response to pralsetinib: a case report.Front Oncol. 2023 Apr 17;13:1078076. doi: 10.3389/fonc.2023.1078076. eCollection 2023. Front Oncol. 2023. PMID: 37139148 Free PMC article.
-
Enhancer-activated RET confers protection against oxidative stress to KMT2A-rearranged acute myeloid leukemia.Cancer Sci. 2024 Mar;115(3):963-973. doi: 10.1111/cas.16069. Epub 2024 Jan 16. Cancer Sci. 2024. PMID: 38226414 Free PMC article.
-
Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments.J Pers Med. 2023 Jul 13;13(7):1132. doi: 10.3390/jpm13071132. J Pers Med. 2023. PMID: 37511745 Free PMC article. Review.
-
First-in-human, phase 1 dose-escalation and dose-expansion study of a RET inhibitor SY-5007 in patients with advanced RET-altered solid tumors.Signal Transduct Target Ther. 2024 Nov 4;9(1):300. doi: 10.1038/s41392-024-02006-9. Signal Transduct Target Ther. 2024. PMID: 39489747 Free PMC article. Clinical Trial.
-
Efficacy and safety of pyrotinib combined with albumin-bound paclitaxel as first-line treatment for HER2-positive metastatic breast cancer in patients previously treated with adjuvant and/or neoadjuvant trastuzumab therapy: The stage 1 results of a single-arm, phase 2 prospective clinical trial.Clin Transl Med. 2024 May;14(5):e1687. doi: 10.1002/ctm2.1687. Clin Transl Med. 2024. PMID: 38738791 Free PMC article. Clinical Trial.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

