Pilot Study of Combination Gemogenovatucel-T (Vigil) and Durvalumab in Women With Relapsed BRCA-wt Triple-Negative Breast or Ovarian Cancer
- PMID: 35957960
- PMCID: PMC9358582
- DOI: 10.1177/11795549221110501
Pilot Study of Combination Gemogenovatucel-T (Vigil) and Durvalumab in Women With Relapsed BRCA-wt Triple-Negative Breast or Ovarian Cancer
Abstract
Background: Gemogenovatucel-T (Vigil) is a triple-function autologous tumor cell immunotherapy which expresses granulocyte-macrophage colony-stimulating factor and decreases expression of furin and downstream TGF-β1 and TGF-β2. Vigil has suggested survival benefit in frontline maintenance ovarian cancer patients who are BRCA-wt. In addition, Vigil demonstrates relapse-free and overall survival advantage in homologous recombination-proficient patients with OC. Further evidence of clinical benefit and safety has been demonstrated in combination with atezolizumab.
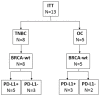
Methods: In this pilot study (NCT02725489), the concurrent combination of the programmed death-ligand 1 (PD-L1) inhibitor durvalumab and Vigil was explored in advanced BRCA-wt relapsed triple-negative breast cancer (TNBC) patients and stage III-IV recurrent/refractory OC patients. Patients received the combination regimen of Vigil (1 × 10e6-10e7 cells/dose intradermally, up to 12 doses) and durvalumab (1500 mg/dose intravenous infusion, up to 12 months) once every 4 weeks. The primary objective was to evaluate safety of this combination. The study included 13 BRCA-wt patients (TNBC, n = 8; OC, n = 5).
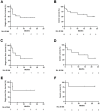
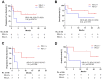
Results: The most common treatment-emergent adverse events (⩾20%) in all patients included injection-site reaction (92.3%), myalgia (38.5%), bruise at injection site (23.1%), and pruritus (23.1%). Three grade 3 treatment-related adverse events were observed and related to durvalumab. There were no grade 4/5 treatment-related adverse events. Median progression-free survival was 7.1 months and the median overall survival was not reached. Prolonged progression-free survival was improved in patients with PD-L1+ tumors (n = 8, hazard ratio = 0.304, 95% confidence interval, 0.0593-1.56, 1-sided P = .04715) compared with those with PD-L1- tumors.
Conclusions: Vigil plus durvalumab was well tolerated and showed promising clinical activity in advanced BRCA-wt TNBC and stage III-IV recurrent/refractory OC patients.
Keywords: Ovarian cancer; Vigil; checkpoint inhibitor; durvalumab; triple-negative breast cancer.
© The Author(s) 2022.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. - PubMed
-
- Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45:27-40 - PubMed
-
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. - PubMed
-
- Spriggs DR, Brady MF, Vaccarello L, et al. Phase III randomized trial of intravenous cisplatin plus a 24- or 96-hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:4466-4471. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

