The O2-sensitive brain stem, hyperoxic hyperventilation, and CNS oxygen toxicity
- PMID: 35957982
- PMCID: PMC9360621
- DOI: 10.3389/fphys.2022.921470
The O2-sensitive brain stem, hyperoxic hyperventilation, and CNS oxygen toxicity
Abstract
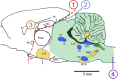
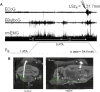
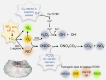
Central nervous system oxygen toxicity (CNS-OT) is a complex disorder that presents, initially, as a sequence of cardio-respiratory abnormalities and nonconvulsive signs and symptoms (S/Sx) of brain stem origin that culminate in generalized seizures, loss of consciousness, and postictal cardiogenic pulmonary edema. The risk of CNS-OT and its antecedent "early toxic indications" are what limits the use of hyperbaric oxygen (HBO2) in hyperbaric and undersea medicine. The purpose of this review is to illustrate, based on animal research, how the temporal pattern of abnormal brain stem responses that precedes an "oxtox hit" provides researchers a window into the early neurological events underlying seizure genesis. Specifically, we focus on the phenomenon of hyperoxic hyperventilation, and the medullary neurons presumed to contribute in large part to this paradoxical respiratory response; neurons in the caudal Solitary complex (cSC) of the dorsomedial medulla, including putative CO2 chemoreceptor neurons. The electrophysiological and redox properties of O2-/CO2-sensitive cSC neurons identified in rat brain slice experiments are summarized. Additionally, evidence is summarized that supports the working hypothesis that seizure genesis originates in subcortical areas and involves cardio-respiratory centers and cranial nerve nuclei in the hind brain (brainstem and cerebellum) based on, respectively, the complex temporal pattern of abnormal cardio-respiratory responses and various nonconvulsive S/Sx that precede seizures during exposure to HBO2.
Keywords: CO2-chemosensitive; O2-sensing; cardiorespiration; hyperbaric oxygen therapy; hyperoxia; seizure; undersea medicine.
Copyright © 2022 Dean and Stavitzski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

