Two small-molecule activators share similar effector sites in the KCNQ1 channel pore but have distinct effects on voltage sensor movements
- PMID: 35957984
- PMCID: PMC9359618
- DOI: 10.3389/fphys.2022.903050
Two small-molecule activators share similar effector sites in the KCNQ1 channel pore but have distinct effects on voltage sensor movements
Abstract
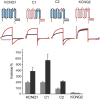
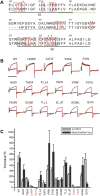
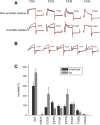
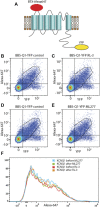
ML277 and R-L3 are two small-molecule activators of KCNQ1, the pore-forming subunit of the slowly activating potassium channel IKs. KCNQ1 loss-of-function mutations prolong cardiac action potential duration and are associated with long QT syndrome, which predispose patients to lethal ventricular arrhythmia. ML277 and R-L3 enhance KCNQ1 current amplitude and slow deactivation. However, the presence of KCNE1, an auxiliary subunit of IKs channels, renders the channel insensitive to both activators. We found that ML277 effects are dependent on several residues in the KCNQ1 pore domain. Some of these residues are also necessary for R-L3 effects. These residues form a putative hydrophobic pocket located between two adjacent KCNQ1 subunits, where KCNE1 subunits are thought to dwell, thus providing an explanation for how KCNE1 renders the IKs channel insensitive to these activators. Our experiments showed that the effect of R-L3 on voltage sensor movement during channel deactivation was much more prominent than that of ML277. Simulations using a KCNQ1 kinetic model showed that the effects of ML277 and R-L3 could be reproduced through two different effects on channel gating: ML277 enhances KCNQ1 channel function through a pore-dependent and voltage sensor-independent mechanism, while R-L3 affects both channel pore and voltage sensor.
Keywords: KCNQ1; agonists; channel; long QT syndrome; voltage sensor.
Copyright © 2022 Chen, Peng, Comollo, Zou, Sampson, Larsson and Kass.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Pharmacological rescue of specific long QT variants of KCNQ1/KCNE1 channels.Front Physiol. 2022 Nov 23;13:902224. doi: 10.3389/fphys.2022.902224. eCollection 2022. Front Physiol. 2022. PMID: 36505078 Free PMC article.
-
Probing binding sites and mechanisms of action of an I(Ks) activator by computations and experiments.Biophys J. 2015 Jan 6;108(1):62-75. doi: 10.1016/j.bpj.2014.10.059. Biophys J. 2015. PMID: 25564853 Free PMC article.
-
Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8732-7. doi: 10.1073/pnas.1300684110. Epub 2013 May 6. Proc Natl Acad Sci U S A. 2013. PMID: 23650380 Free PMC article.
-
Gating and Regulation of KCNQ1 and KCNQ1 + KCNE1 Channel Complexes.Front Physiol. 2020 Jun 4;11:504. doi: 10.3389/fphys.2020.00504. eCollection 2020. Front Physiol. 2020. PMID: 32581825 Free PMC article. Review.
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
Cited by
-
Integrative analysis of KCNQ1 variants reveals molecular mechanisms of type 1 long QT syndrome pathogenesis.Proc Natl Acad Sci U S A. 2025 Feb 25;122(8):e2412971122. doi: 10.1073/pnas.2412971122. Epub 2025 Feb 19. Proc Natl Acad Sci U S A. 2025. PMID: 39969993 Free PMC article.
-
Different fluorescent labels report distinct components of spHCN channel voltage sensor movement.J Gen Physiol. 2024 Aug 5;156(8):e202413559. doi: 10.1085/jgp.202413559. Epub 2024 Jul 5. J Gen Physiol. 2024. PMID: 38968404 Free PMC article.
-
Pharmacological rescue of specific long QT variants of KCNQ1/KCNE1 channels.Front Physiol. 2022 Nov 23;13:902224. doi: 10.3389/fphys.2022.902224. eCollection 2022. Front Physiol. 2022. PMID: 36505078 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

