Circadian Oscillations in the Murine Preoptic Area Are Reset by Temperature, but Not Light
- PMID: 35957988
- PMCID: PMC9361018
- DOI: 10.3389/fphys.2022.934591
Circadian Oscillations in the Murine Preoptic Area Are Reset by Temperature, but Not Light
Abstract
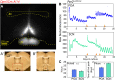
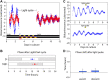
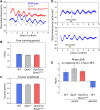
Mammals maintain their internal body temperature within a physiologically optimal range. This involves the regulation of core body temperature in response to changing environmental temperatures and a natural circadian oscillation of internal temperatures. The preoptic area (POA) of the hypothalamus coordinates body temperature by responding to both external temperature cues and internal brain temperature. Here we describe an autonomous circadian clock system in the murine ventromedial POA (VMPO) in close proximity to cells which express the atypical violet-light sensitive opsin, Opn5. We analyzed the light-sensitivity and thermal-sensitivity of the VMPO circadian clocks ex vivo. The phase of the VMPO circadian oscillations was not influenced by light. However, the VMPO clocks were reset by temperature changes within the physiological internal temperature range. This thermal-sensitivity of the VMPO circadian clock did not require functional Opn5 expression or a functional circadian clock within the Opn5-expressing cells. The presence of temperature-sensitive circadian clocks in the VMPO provides an advancement in the understanding of mechanisms involved in the dynamic regulation of core body temperature.
Keywords: Opn5; circadian rhythm; neuropsin; preoptic area; temperature.
Copyright © 2022 Díaz, Gordon, Lang and Buhr.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Diurnal regulation of metabolism by Gs-alpha in hypothalamic QPLOT neurons.PLoS One. 2023 May 4;18(5):e0284824. doi: 10.1371/journal.pone.0284824. eCollection 2023. PLoS One. 2023. PMID: 37141220 Free PMC article.
-
Feeding regime synchronizes circadian clock in choroid plexus - insight into a complex mechanism.Cell Mol Life Sci. 2025 Jun 23;82(1):247. doi: 10.1007/s00018-025-05798-3. Cell Mol Life Sci. 2025. PMID: 40548998 Free PMC article.
-
Later eating timing in relation to an individual internal clock is associated with lower insulin sensitivity and affected by genetic factors.EBioMedicine. 2025 Jun;116:105737. doi: 10.1016/j.ebiom.2025.105737. Epub 2025 Apr 29. EBioMedicine. 2025. PMID: 40305967 Free PMC article. Clinical Trial.
-
A review on modeling approaches for the transcriptional regulatory network intricacies of circadian clock genes in plants.Planta. 2025 Jun 5;262(1):17. doi: 10.1007/s00425-025-04735-9. Planta. 2025. PMID: 40471439 Review.
-
Circadian Rhythm Resynchronization as a Targeted Intervention for Mood Stability in Bipolar Disorder.S D Med. 2025 May;78(suppl 5):s33. S D Med. 2025. PMID: 40550173 Review.
Cited by
-
Diurnal regulation of metabolism by Gs-alpha in hypothalamic QPLOT neurons.PLoS One. 2023 May 4;18(5):e0284824. doi: 10.1371/journal.pone.0284824. eCollection 2023. PLoS One. 2023. PMID: 37141220 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

