Assessment of the control measures of the Category A diseases of the Animal Health Law: prohibitions in restricted zones and risk-mitigating treatments for products of animal origin and other materials
- PMID: 35958104
- PMCID: PMC9361132
- DOI: 10.2903/j.efsa.2022.7443
Assessment of the control measures of the Category A diseases of the Animal Health Law: prohibitions in restricted zones and risk-mitigating treatments for products of animal origin and other materials
Abstract
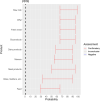
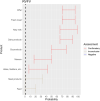
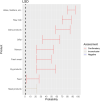
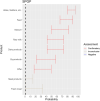
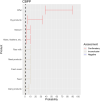
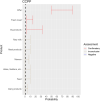
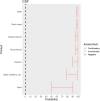
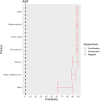
EFSA received a mandate from the European Commission to assess the effectiveness of prohibitions of certain activities in restricted zones, and of certain risk mitigation treatments for products of animal origin and other materials with respect to diseases included in the Category A list in the Animal Health Law (Regulation (EU) 2016/429). This opinion belongs to a series of opinions where other disease-specific control measures have been assessed. In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of (i) prohibiting the movements of certain products, notably germinal products (semen, oocytes, embryos and hatching eggs), products of animal origin and animal by-products and feed of plant origin, hay and straw, and (ii) risk mitigation treatments for products of animal origin. In terms of semen, oocytes, embryos and hatching eggs, it was agreed that there was a lack of evidence particularly for embryos and oocytes reflected in a varying degree of uncertainty, whether these commodities could potentially contain the pathogen under consideration. The scenario assessed did not consider whether the presence of pathogen would lead to infection in the recipient animal. In terms of animal products, certain animal by-products and movement of feed of plant origin and straw, the assessment considered the ability of the commodity to transmit disease to another animal if exposed. For most pathogens, products were to some degree considered a risk, but lack of field evidence contributed to the uncertainty, particularly as potential exposure of ruminants to meat products is concerned. In terms of the risk mitigating treatments, recommendations have been made for several of these treatments, because the treatment description is not complete, the evidence is poor or inconclusive, or the evidence points to the treatment being ineffective.
Keywords: Category A diseases; animal products; control measures; germinal products; movement prohibitions; risk‐mitigating treatments.
© 2022 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures
Similar articles
-
Assessment of the control measures of the category A diseases of Animal Health Law: peste des petits ruminants.EFSA J. 2021 Jul 30;19(7):e06708. doi: 10.2903/j.efsa.2021.6708. eCollection 2021 Jul. EFSA J. 2021. PMID: 34354766 Free PMC article.
-
Assessment of the control measures of the category A diseases of Animal Health Law: Newcastle disease.EFSA J. 2021 Dec 2;19(12):e06946. doi: 10.2903/j.efsa.2021.6946. eCollection 2021 Dec. EFSA J. 2021. PMID: 34900005 Free PMC article.
-
Assessment of the control measures of the category A diseases of Animal Health Law: Rift Valley Fever.EFSA J. 2022 Jan 19;20(1):e07070. doi: 10.2903/j.efsa.2022.7070. eCollection 2022 Jan. EFSA J. 2022. PMID: 35079289 Free PMC article.
-
The risks posed by the importation of animals vaccinated against foot and mouth disease and products derived from vaccinated animals: a review.Rev Sci Tech. 2003 Dec;22(3):823-35. doi: 10.20506/rst.22.3.1435. Rev Sci Tech. 2003. PMID: 15005540 Review.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
Cited by
-
Assessment on the efficacy of methods 2 to 5 and method 7 set out in Commission Regulation (EU) No 142/2011 to inactivate relevant pathogens when producing processed animal protein of porcine origin intended to feed poultry and aquaculture animals.EFSA J. 2023 Jul 5;21(7):e08093. doi: 10.2903/j.efsa.2023.8093. eCollection 2023 Jul. EFSA J. 2023. PMID: 37416785 Free PMC article.
References
-
- Cardona C, Wileman B, Malladi S, Ceballos R, Culhane M, Munoz‐Aguayo J, Flores‐Figueroa C, Halvorson D, Walz E, St. Charles K and Bonney P, 2021. The risk of highly pathogenic influenza A virus transmission to turkey hen flocks through artificial insemination. Avian Diseases, 65, 303–309. 10.1637/aviandiseases-D-20-00132 - DOI - PubMed
LinkOut - more resources
Full Text Sources
