Mycoplasma bovis inhibits autophagy in bovine mammary epithelial cells via a PTEN/PI3K-Akt-mTOR-dependent pathway
- PMID: 35958147
- PMCID: PMC9360976
- DOI: 10.3389/fmicb.2022.935547
Mycoplasma bovis inhibits autophagy in bovine mammary epithelial cells via a PTEN/PI3K-Akt-mTOR-dependent pathway
Abstract
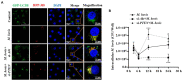
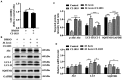
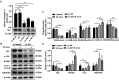
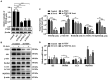
Although autophagy can eliminate some intracellular pathogens, others, e.g., Staphylococcus aureus, Salmonella, Mycoplasma bovis, can evade it. The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway, a key regulator of autophagy, is involved in initiation and promotion of a range of pathological diseases. As the effects of M. bovis on the autophagic pathway are not well documented, our objective was to elucidate the effects of M. bovis infection on the PI3K-Akt-mTOR cellular autophagic pathway in bovine mammary epithelial cells (bMECs). Ultrastructure of bMECs infected with M. bovis was assessed with transmission electron microscopy, co-localization of LC3 puncta with M. bovis was confirmed by laser confocal microscopy, and autophagy-related indicators were quantified with Western blotting and RT-PCR. In M. bovis-infected bMECs, intracellular M. bovis was encapsulated by membrane-like structures, the expression level of LC3-II and Beclin1 protein decreased at the middle stage of infection, degradation of SQSTM1/P62 was blocked, autophagy of bMECs was inhibited, and PI3K-Akt-mTOR protein was activated by phosphorylation. Furthermore, the tumor suppressor PTEN can inhibit the PI3K-Akt signaling pathway through dephosphorylation of phosphatidylinositol 3,4,5-trisphosphate and may be important for cellular resistance to infection. In the present study, the number of intracellular M. bovis was inversely related to the change in the level of autophagy markers (e.g., LC3-II, SQSTM1/P62) within host cells induced by the low knockdown of Akt or PTEN. We concluded that M. bovis-infected bMECs alleviated cellular autophagy through a PI3K-Akt-mTOR pathway, and that PTEN acted as a protective gene regulating autophagy, a key step in controlling infection.
Keywords: Mycoplasma bovis; PI3K-Akt-mTOR pathway; PTEN; autophagy; bovine mammary epithelial cells.
Copyright © 2022 Xu, Liu, Mayinuer, Lin, Wang, Gao, Wang, Kastelic and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prototheca bovis induces autophagy in bovine mammary epithelial cells via the HIF-1α and AMPKα/ULK1 pathway.Front Immunol. 2022 Sep 2;13:934819. doi: 10.3389/fimmu.2022.934819. eCollection 2022. Front Immunol. 2022. PMID: 36148236 Free PMC article.
-
Mycoplasma bovis subverts autophagy to promote intracellular replication in bovine mammary epithelial cells cultured in vitro.Vet Res. 2021 Oct 14;52(1):130. doi: 10.1186/s13567-021-01002-z. Vet Res. 2021. PMID: 34649594 Free PMC article.
-
Streptococcus agalactiae-induced autophagy of bovine mammary epithelial cell via PI3K/AKT/mTOR pathway.J Dairy Res. 2022 Apr 7:1-7. doi: 10.1017/S0022029922000243. Online ahead of print. J Dairy Res. 2022. PMID: 35388773
-
The Role of mTOR in Mycobacterium tuberculosis Infection.Biomedicines. 2024 Oct 1;12(10):2238. doi: 10.3390/biomedicines12102238. Biomedicines. 2024. PMID: 39457551 Free PMC article. Review.
-
The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets.Front Cardiovasc Med. 2023 Mar 29;10:1088575. doi: 10.3389/fcvm.2023.1088575. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37063954 Free PMC article. Review.
Cited by
-
High Concentration of FBS Can Save mTOR Down-Regulation Caused by Mycoplasmas bovis Infection.Vet Sci. 2022 Nov 11;9(11):630. doi: 10.3390/vetsci9110630. Vet Sci. 2022. PMID: 36423079 Free PMC article.
-
Estrogen promotes autophagy in the mammary epithelial cells of dairy sheep via the CXCL12/CXCR4 axis.J Anim Sci. 2025 Jan 4;103:skaf064. doi: 10.1093/jas/skaf064. J Anim Sci. 2025. PMID: 40341494 Free PMC article.
-
Molecular mechanism of Dang-Shen-Yu-Xing decoction against Mycoplasma bovis pneumonia based on network pharmacology, molecular docking, molecular dynamics simulations and experimental verification.Front Vet Sci. 2024 Sep 24;11:1431233. doi: 10.3389/fvets.2024.1431233. eCollection 2024. Front Vet Sci. 2024. PMID: 39380772 Free PMC article.
-
Alteration in Tracheal Morphology and Transcriptomic Features in Calves After Infection with Mycoplasma bovis.Microorganisms. 2025 Feb 18;13(2):442. doi: 10.3390/microorganisms13020442. Microorganisms. 2025. PMID: 40005807 Free PMC article.
-
Antioxidative Sirt1 and the Keap1-Nrf2 Signaling Pathway Impair Inflammation and Positively Regulate Autophagy in Murine Mammary Epithelial Cells or Mammary Glands Infected with Streptococcus uberis.Antioxidants (Basel). 2024 Jan 29;13(2):171. doi: 10.3390/antiox13020171. Antioxidants (Basel). 2024. PMID: 38397769 Free PMC article.
References
-
- Chia Y. C., Catimel B., Lio D. S., Ang C. S., Peng B., Wu H., et al. . (2015). The C-terminal tail inhibitory phosphorylation sites of PTEN regulate its intrinsic catalytic activity and the kinetics of its binding to phosphatidylinositol-4,5-bisphosphate. Arch. Biochem. Biophys. 587, 48–60. doi: 10.1016/j.abb.2015.10.004, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

