Effects of compound small peptides of Chinese medicine on intestinal immunity and cecal intestinal flora in CTX immunosuppressed mice
- PMID: 35958151
- PMCID: PMC9358959
- DOI: 10.3389/fmicb.2022.959726
Effects of compound small peptides of Chinese medicine on intestinal immunity and cecal intestinal flora in CTX immunosuppressed mice
Abstract
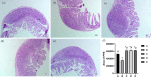
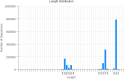
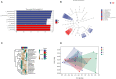
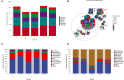
The study was designed to explore the improvement effect of CSPCM (compound small peptide of Chinese medicine) on intestinal immunity and microflora through the treatment of different doses of CSPCM. A total of 100 male Kunming mice were weighed and divided into five groups, namely, group A (control group), group B (model group), group C (0.1 g/kg·bw CSPCM), group D (0.2 g/kg·bw CSPCM), and group E (0.4 g/kg·bw CSPCM). The use of CTX (cyclophosphamide) caused a series of negative effects: the secretion of IL-2, IL-22, TNF-α, sIgA, length of the villi, and the area of Pey's node were significantly reduced (P < 0.05); the depth of crypt and the percent of CD3+ and CD4+ cells were significantly increased (P < 0.05); the cecal flora taxa decreased; the abundance of Firmicutes and Lactobacillus increased; and the abundance of Bacteroidetes, Deferribacteres, Proteobacteria, Mucispirillum, Bacteroides, and Flexisprra decreased. The addition of CSPCM improved the secretion of cytokines and the development of intestinal villi, crypts, and Pey's node. The number of CD3+ and CD4+ cells in groups C, D, and E was significantly higher than that in group B (P < 0.05). Compared with group B, the abundance of Firmicutes in groups C, D, and E was decreased, and the Bacteroidetes, Deferribacteres, and Proteobacteria increased. The abundance of Lactobacillus decreased, while that of Mucispirillum, Bacteroides, and Flexisprra increased. It is concluded that cyclophosphamide is extremely destructive to the intestinal area and has a great negative impact on the development of the small intestine, the intestinal immune system, and the intestinal flora. The CSPCM can improve the negative effects of CTX.
Keywords: Chinese medicine; immune; mice; peptide; small intestine.
Copyright © 2022 Cui, Zhang, Lu, Dou, Jiao, Bao and Shi.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

