Cross-kingdom co-occurrence networks in the plant microbiome: Importance and ecological interpretations
- PMID: 35958158
- PMCID: PMC9358436
- DOI: 10.3389/fmicb.2022.953300
Cross-kingdom co-occurrence networks in the plant microbiome: Importance and ecological interpretations
Abstract
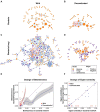
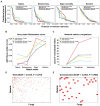
Microbial co-occurrence network analysis is being widely used for data exploration in plant microbiome research. Still, challenges lie in how well these microbial networks represent natural microbial communities and how well we can interpret and extract eco-evolutionary insights from the networks. Although many technical solutions have been proposed, in this perspective, we touch on the grave problem of kingdom-level bias in network representation and interpretation. We underscore the eco-evolutionary significance of using cross-kingdom (bacterial-fungal) co-occurrence networks to increase the network's representability of natural communities. To do so, we demonstrate how ecosystem-level interpretation of plant microbiome evolution changes with and without multi-kingdom analysis. Then, to overcome oversimplified interpretation of the networks stemming from the stereotypical dichotomy between bacteria and fungi, we recommend three avenues for ecological interpretation: (1) understanding dynamics and mechanisms of co-occurrence networks through generalized Lotka-Volterra and consumer-resource models, (2) finding alternative ecological explanations for individual negative and positive fungal-bacterial edges, and (3) connecting cross-kingdom networks to abiotic and biotic (host) environments.
Keywords: cross-kingdom interaction; ecological framework; fungi; network; plant microbiome.
Copyright © 2022 Lee, Kim and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ballhausen M. B., de Boer W. (2016). The sapro-rhizosphere: carbon flow from saprotrophic fungi into fungus-feeding bacteria. Soil Biol. Biochem. 102, 14–17. doi: 10.1016/j.soilbio.2016.06.014 - DOI
-
- Banerjee S., Kirkby C. A., Schmutter D., Bissett A., Kirkegaard J. A., Richardson A. E. (2016). Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 97, 188–198. doi: 10.1016/j.soilbio.2016.03.017 - DOI
Publication types
LinkOut - more resources
Full Text Sources

