Species-Specific Expression of Growth-Regulatory Genes in 2 Anoles with Divergent Patterns of Sexual Size Dimorphism
- PMID: 35958165
- PMCID: PMC9362763
- DOI: 10.1093/iob/obac025
Species-Specific Expression of Growth-Regulatory Genes in 2 Anoles with Divergent Patterns of Sexual Size Dimorphism
Abstract
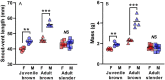
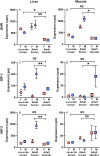
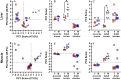
Sexual size dimorphism is widespread in nature and often develops through sexual divergence in growth trajectories. In vertebrates, the growth hormone/insulin-like growth factor (GH/IGF) network is an important regulator of growth, and components of this network are often regulated in sex-specific fashion during the development of sexual size dimorphism. However, expression of the GH/IGF network is not well characterized outside of mammalian model systems, and the extent to which species differences in sexual size dimorphism are related to differences in GH/IGF network expression is unclear. To begin bridging this gap, we compared GH/IGF network expression in liver and muscle from 2 lizard congeners, one with extreme male-biased sexual size dimorphism (brown anole, Anolis sagrei), and one that is sexually monomorphic in size (slender anole, A. apletophallus). Specifically, we tested whether GH/IGF network expression in adult slender anoles resembles the highly sex-biased expression observed in adult brown anoles or the relatively unbiased expression observed in juvenile brown anoles. We found that adults of the 2 species differed significantly in the strength of sex-biased expression for several key upstream genes in the GH/IGF network, including insulin-like growth factors 1 and 2. However, species differences in sex-biased expression were minor when comparing adult slender anoles to juvenile brown anoles. Moreover, the multivariate expression of the entire GH/IGF network (as represented by the first two principal components describing network expression) was sex-biased for the liver and muscle of adult brown anoles, but not for either tissue in juvenile brown anoles or adult slender anoles. Our work suggests that species differences in sex-biased expression of genes in the GH/IGF network (particularly in the liver) may contribute to the evolution of species differences in sexual size dimorphism.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology.
Figures
Similar articles
-
Sex-specific microhabitat use is associated with sex-biased thermal physiology in Anolis lizards.J Exp Biol. 2021 Jan 22;224(Pt 2):jeb235697. doi: 10.1242/jeb.235697. J Exp Biol. 2021. PMID: 33328289
-
Sex-Specific Population Differences in Resting Metabolism Are Associated with Intraspecific Variation in Sexual Size Dimorphism of Brown Anoles.Physiol Biochem Zool. 2021 Jul-Aug;94(4):205-214. doi: 10.1086/714638. Physiol Biochem Zool. 2021. PMID: 33970831
-
Ontogenetic Change in Male Expression of Testosterone-Responsive Genes Contributes to the Emergence of Sex-Biased Gene Expression in Anolis sagrei.Front Physiol. 2022 Jun 2;13:886973. doi: 10.3389/fphys.2022.886973. eCollection 2022. Front Physiol. 2022. PMID: 35721538 Free PMC article.
-
Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies.Growth Horm IGF Res. 2016 Apr;27:7-17. doi: 10.1016/j.ghir.2015.12.004. Epub 2015 Dec 31. Growth Horm IGF Res. 2016. PMID: 26843472 Free PMC article. Review.
-
Growth hormone and the insulin-like growth factor system in myogenesis.Endocr Rev. 1996 Oct;17(5):481-517. doi: 10.1210/edrv-17-5-481. Endocr Rev. 1996. PMID: 8897022 Review.
Cited by
-
Differential Mitochondrial Genome Expression of Four Skink Species Under High-Temperature Stress and Selection Pressure Analyses in Scincidae.Animals (Basel). 2025 Mar 30;15(7):999. doi: 10.3390/ani15070999. Animals (Basel). 2025. PMID: 40218392 Free PMC article.
-
Sex-specific effects of dietary restriction on physiological variables in Japanese quails.Ecol Evol. 2024 May 23;14(5):e11405. doi: 10.1002/ece3.11405. eCollection 2024 May. Ecol Evol. 2024. PMID: 38799393 Free PMC article.
References
-
- Andrews R, Rand AS. 1974. Reproductive effort in anoline lizards. Ecol 1317–27.
-
- Andrews RM. 1976. Growth rate in island and mainland anoline lizards. Copeia 477–82.
-
- Andrews RM. 1979. Reproductive effort of female Anolis limifrons (Sauria: Iguanidae). Copeia 620–6.
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
