Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial
- PMID: 35958172
- PMCID: PMC9357919
- DOI: 10.3389/fped.2022.943529
Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial
Abstract
Background: Some studies suggested that adequate levels of vitamin D (VD) decrease the risk of severe COVID-19. Information about the effectiveness of VD supplementation in children is scarce.
Objective: To assess the efficacy and safety of VD supplementation compared to the standard of care in hospitalized children with COVID-19.
Patients and methods: An open-label randomized controlled single-blind clinical trial was carried out. We included patients from 1 month to 17 years, with moderate COVID-19, who required hospitalization and supplemental oxygen. They were randomized into two groups: the VD group, which received doses of 1,000 (children < 1 year) or 2,000 IU/day (from 1 to 17 years) and the group without VD (control). The outcome variables were the progression of oxygen requirement, the development of complications, and death.
Statistical analysis: For comparison between groups, we used the chi-squared test or Fisher's exact test and the Mann-Whitney U test. Absolute risk reduction (ARR) and the number needed to treat (NNT) were calculated. p ≤ 0.05 was considered statistically significant.
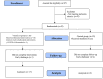
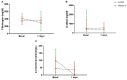
Results: From 24 March 2020 to 31 March 2021, 87 patients were eligible to participate in the trial; 45 patients were randomized: 20 to the VD group and 25 to the control group. There was no difference in general characteristics at baseline, including serum VD levels (median 13.8 ng/ml in the VD group and 11.4 ng/ml in the control group).
Outcomes: 2/20 (10%) in the VD group vs. 9/25 (36%) in the control group progressed to a superior ventilation modality (p = 0.10); one patient in the VD group died (5%) compared to 6 (24%) patients in the control group (p = 0.23). ARR was 26% (95% CI 8.8 to 60.2%) and NNT was 3 (2 to 11) for progression and ARR was 19% (95% CI -3.9 to 42.8%) and NNT was 6 (2 to 26) for death. None of the patients receiving VD had adverse effects. The trial was stopped for ethical reasons; since after receiving the results of the basal VD values, none of the patients had normal levels.
Conclusion: In this trial, VD supplementation in pediatric patients seems to decrease the risk of COVID-19 progression and death. More studies are needed to confirm these findings.
Clinical trial registration: This protocol was registered on ClinicalTrials.gov with the registration number NCT04502667.
Keywords: COVID-19; Latin America; Pediatrics; SARS-CoV-2; children; mortality; vitamin D.
Copyright © 2022 Zurita-Cruz, Fonseca-Tenorio, Villasís-Keever, López-Alarcón, Parra-Ortega, López-Martínez and Miranda-Novales.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Effect of dexamethasone in patients with ARDS and COVID-19 - prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 1;22(1):172. doi: 10.1186/s13063-021-05116-9. Trials. 2021. PMID: 33648568 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Unlocking Vitality: A Comprehensive Review of Vitamin D's Impact on Clinical Outcomes in Critically Ill Children.Cureus. 2024 May 22;16(5):e60840. doi: 10.7759/cureus.60840. eCollection 2024 May. Cureus. 2024. PMID: 38910623 Free PMC article. Review.
Cited by
-
Mechanistic Insight into the role of Vitamin D and Zinc in Modulating Immunity Against COVID-19: A View from an Immunological Standpoint.Biol Trace Elem Res. 2023 Dec;201(12):5546-5560. doi: 10.1007/s12011-023-03620-4. Epub 2023 Mar 9. Biol Trace Elem Res. 2023. PMID: 36890344 Free PMC article. Review.
-
Therapeutic effects of vitamin D supplementation on COVID-19 aggravation: a systematic review and meta-analysis of randomized controlled trials.Front Pharmacol. 2024 May 27;15:1367686. doi: 10.3389/fphar.2024.1367686. eCollection 2024. Front Pharmacol. 2024. PMID: 38860175 Free PMC article.
-
Possible Impact of Vitamin D Status and Supplementation on SARS-CoV-2 Infection Risk and COVID-19 Symptoms in a Cohort of Patients with Inflammatory Bowel Disease.Nutrients. 2022 Dec 29;15(1):169. doi: 10.3390/nu15010169. Nutrients. 2022. PMID: 36615826 Free PMC article.
-
Zika virus infection suppresses CYP24A1 and CAMP expression in human monocytes.Arch Virol. 2024 Jun 6;169(7):135. doi: 10.1007/s00705-024-06050-2. Arch Virol. 2024. PMID: 38839691 Free PMC article.
-
COVID-19 Biomarkers Comparison: Children, Adults and Elders.Medicina (Kaunas). 2023 May 3;59(5):877. doi: 10.3390/medicina59050877. Medicina (Kaunas). 2023. PMID: 37241109 Free PMC article.
References
-
- COVID 19 en Pediatría: Valoración Crítica de la Evidencia . Comité/Grupo de Pediatría Basada en la Evidencia de la AEP y AEPap. (2022). Available online at https://www.aeped.es/comite-pediatria-basada-en-evidencia/documentos/cov... (accesed March 10, 2022).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous