ABCG11 modulates cytokinin responses in Arabidopsis thaliana
- PMID: 35958217
- PMCID: PMC9358225
- DOI: 10.3389/fpls.2022.976267
ABCG11 modulates cytokinin responses in Arabidopsis thaliana
Abstract
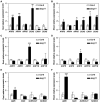
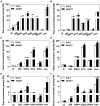
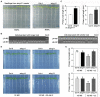
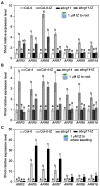
The Arabidopsis ABC transporter ABCG11 transports lipidic precursors of surface coating polymers at the plasma membrane of epidermal cells. Mutants in ABCG11 exhibit severe developmental defects, suggesting that ABCG11 might also participate in phytohormone-mediated development. Here, we report that ABCG11 is involved in cytokinin-mediated development. The roots of abcg11 mutant seedlings failed to respond to cytokinins and accumulated more cytokinins than wild-type roots. When grown under short-day conditions, abcg11 exhibited longer roots and shorter hypocotyls compared to wild type, similar to abcg14, a knockout mutant in a cytokinin transporter. Treatment with exogenous trans-zeatin, which inhibits primary root elongation in the wild type, enhanced abcg11 primary root elongation. It also increased the expression of cytokinin-responsive Arabidopsis response regulator (ARR) genes, and the signal of the TCS::GFP reporter in abcg11 roots compared to wild-type roots, suggesting that cytokinin signaling was enhanced in abcg11 roots. When we treated only the roots of abcg11 with trans-zeatin, their shoots showed lower ARR induction than the wild type. The abcg14 abcg11 double mutant did not have additional root phenotypes compared to abcg11. Together, these results suggest that ABCG11 is necessary for normal cytokinin-mediated root development, likely because it contributes to cytokinin transport, either directly or indirectly.
Keywords: ABC transporters; ABCG11; ABCG14; heterodimer; phytohormone; root; signaling; transport.
Copyright © 2022 Yang, Zhang, Kojima, Takebayashi, Uragami, Kiba, Sakakibara and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Limitation of Cytokinin Export to the Shoots by Nucleoside Transporter ENT3 and its Linkage with Root Elongation in Arabidopsis.Cells. 2021 Feb 8;10(2):350. doi: 10.3390/cells10020350. Cells. 2021. PMID: 33567681 Free PMC article.
-
Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin.Proc Natl Acad Sci U S A. 2014 May 13;111(19):7150-5. doi: 10.1073/pnas.1321519111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778257 Free PMC article.
-
Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana.Plant Cell Physiol. 2007 Jan;48(1):84-96. doi: 10.1093/pcp/pcl040. Epub 2006 Nov 27. Plant Cell Physiol. 2007. PMID: 17132632
-
Multiple routes communicating nitrogen availability from roots to shoots: a signal transduction pathway mediated by cytokinin.J Exp Bot. 2002 Apr;53(370):971-7. doi: 10.1093/jexbot/53.370.971. J Exp Bot. 2002. PMID: 11912239 Review.
-
The role of ABCG-type ABC transporters in phytohormone transport.Biochem Soc Trans. 2015 Oct;43(5):924-30. doi: 10.1042/BST20150106. Biochem Soc Trans. 2015. PMID: 26517905 Free PMC article. Review.
Cited by
-
The cytokinin efflux transporter ABCC4 participates in Arabidopsis root system development.Plant Physiol. 2024 Dec 24;197(1):kiae628. doi: 10.1093/plphys/kiae628. Plant Physiol. 2024. PMID: 39719052 Free PMC article.
-
Arabidopsis ABCG14 forms a homodimeric transporter for multiple cytokinins and mediates long-distance transport of isopentenyladenine-type cytokinins.Plant Commun. 2023 Mar 13;4(2):100468. doi: 10.1016/j.xplc.2022.100468. Epub 2022 Oct 28. Plant Commun. 2023. PMID: 36307987 Free PMC article.
-
Transcriptomic Analysis of Alternative Splicing Events during Different Fruit Ripening Stages of Coffea arabica L.Genes (Basel). 2024 Apr 5;15(4):459. doi: 10.3390/genes15040459. Genes (Basel). 2024. PMID: 38674393 Free PMC article.
-
Cytokinin: From autoclaved DNA to two-component signaling.Plant Cell. 2024 May 1;36(5):1429-1450. doi: 10.1093/plcell/koad327. Plant Cell. 2024. PMID: 38163638 Free PMC article. Review.
-
Insights on Phytohormonal Crosstalk in Plant Response to Nitrogen Stress: A Focus on Plant Root Growth and Development.Int J Mol Sci. 2023 Feb 11;24(4):3631. doi: 10.3390/ijms24043631. Int J Mol Sci. 2023. PMID: 36835044 Free PMC article. Review.
References
-
- Anderson J. P., Badruzsaufari E., Schenk P. M., Manners J. M., Desmond O. J., Ehlert C., et al. . (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16, 3460–3479. doi: 10.1105/tpc.104.025833, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

