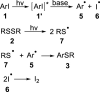Metal-free photo-induced sulfidation of aryl iodide and other chalcogenation
- PMID: 35958235
- PMCID: PMC9360480
- DOI: 10.3389/fchem.2022.941016
Metal-free photo-induced sulfidation of aryl iodide and other chalcogenation
Abstract
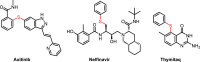
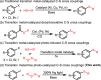
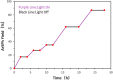
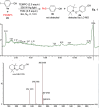
A photo-induced C-S radical cross-coupling of aryl iodides and disulfides under transition-metal and external photosensitizer free conditions for the synthesis of aryl sulfides at room temperature has been presented, which features mild reaction conditions, broad substrate scope, high efficiency, and good functional group compatibility. The developed methodology could be readily applied to forge C-S bond in the field of pharmaceutical and material science.
Keywords: aryl sulfides; cross-coupling; disulfides; metal-free; photo-induced.
Copyright © 2022 Mao, Zhao, Luo, Wang, Yuan, Hu, Hu, Zhang, Ye, Wang and Chen.
Conflict of interest statement
Author ZL was employed by Xi’an Changqing Chemical Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Amiri K., Rostami A., Rostami A. (2016b). CuFe 2 O 4 magnetic nanoparticle catalyzed odorless synthesis of sulfides using phenylboronic acid and aryl halides in the presence of S 8. New J. Chem. 40, 7522–7528. 10.1039/c6nj01434h - DOI
-
- Amiri K., Rostami A., Samadi S., Rostami A. (2016a). Cu-ZSM5 as reusable catalyst for the one-pot, odorless and ligand-free C-S bond formation. Catal. Commun. 86, 108–112. 10.1016/j.catcom.2016.07.019 - DOI
-
- Atashkar B., Rostami A., Rostami A., Zolfigol M. A. (2019). NiFe2O4 as a magnetically recoverable nanocatalyst for odourless C–S bond formation via the cleavage of C–O bond in the presence of S8 under mild and green conditions. Appl. Organomet. Chem. 33, e4691. 10.1002/aoc.4691 - DOI
LinkOut - more resources
Full Text Sources