Heat Shock Protein 70 Mediates the Protective Effect of Naringenin on High-Glucose-Induced Alterations of Endothelial Function
- PMID: 35958293
- PMCID: PMC9359828
- DOI: 10.1155/2022/7275765
Heat Shock Protein 70 Mediates the Protective Effect of Naringenin on High-Glucose-Induced Alterations of Endothelial Function
Abstract
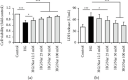
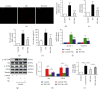
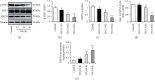
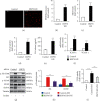
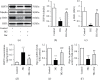
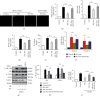
Endothelial dysfunction plays a pivotal role in the development and progression of diabetic vascular complications. Naringenin (Nar) is a flavanone bioactive isolated from citrus fruits known to have in vitro and in vivo antidiabetic properties. However, whether Nar affects endothelial function remains unclear in diabetes or under high-glucose (HG) condition. Using an in vitro model of hyperglycemia in human umbilical vein endothelial cells (HUVECs), we found that Nar administration markedly attenuated HG-induced alterations of endothelial function, evidenced by the mitigation of oxidative stress and inflammation, the reduction of cell adhesion molecular expressions, and the improvement of insulin resistance. We also found that HG exposure significantly reduced the levels of intracellular heat shock protein 70 (iHSP70 or iHSPA1A) and the release of HSP70 from HUVECs. HSP70 depletion mimicked and clearly diminished the protective effects of Nar on HG-induced alterations of endothelial function. In addition, Nar treatment significantly enhanced iHSP70 protein levels through a transcription-dependent manner. These results demonstrated that Nar could protect HUVECs against HG-induced alterations of endothelial function through upregulating iHSP70 protein levels. These findings are also helpful in providing new therapeutic strategies that are promising in the clinical use of Nar for the treatment of diabetes and diabetic complications.
Copyright © 2022 Zhihan Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Figures
References
-
- Shi Y., Vanhoutte P. M. Macro- and microvascular endothelial dysfunction in diabetes. Journal of Diabetes . 2017;9(5):434–449. - PubMed
-
- Wołoszyn-Durkiewicz A., Myśliwiec M. The prognostic value of inflammatory and vascular endothelial dysfunction biomarkers in microvascular and macrovascular complications in type 1 diabetes. Pediatric Endocrinology, Diabetes and Metabolism . 2019;25(1):28–35. - PubMed
LinkOut - more resources
Full Text Sources

