Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors
- PMID: 35958418
- PMCID: PMC9360326
- DOI: 10.3389/fcvm.2022.931475
Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors
Abstract
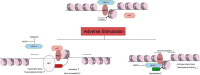
Cardiac hypertrophy is a key process in cardiac remodeling development, leading to ventricle enlargement and heart failure. Recently, studies show the complicated relation between cardiac hypertrophy and epigenetic modification. Post-translational modification of histone is an essential part of epigenetic modification, which is relevant to multiple cardiac diseases, especially in cardiac hypertrophy. There is a group of enzymes related in the balance of histone acetylation/deacetylation, which is defined as histone acetyltransferase (HAT) and histone deacetylase (HDAC). In this review, we introduce an important enzyme family HDAC, a key regulator in histone deacetylation. In cardiac hypertrophy HDAC I downregulates the anti-hypertrophy gene expression, including Kruppel-like factor 4 (Klf4) and inositol-5 phosphatase f (Inpp5f), and promote the development of cardiac hypertrophy. On the contrary, HDAC II binds to myocyte-specific enhancer factor 2 (MEF2), inhibit the assemble ability to HAT and protect against cardiac hypertrophy. Under adverse stimuli such as pressure overload and calcineurin stimulation, the HDAC II transfer to cytoplasm, and MEF2 can bind to nuclear factor of activated T cells (NFAT) or GATA binding protein 4 (GATA4), mediating inappropriate gene expression. HDAC III, also known as SIRTs, can interact not only to transcription factors, but also exist interaction mechanisms to other HDACs, such as HDAC IIa. We also present the latest progress of HDAC inhibitors (HDACi), as a potential treatment target in cardiac hypertrophy.
Keywords: cardiac hypertrophy; epigenetics; gene regulation; histone deacetylase; small molecule inhibitors.
Copyright © 2022 Han, Nie, Wang and Ni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy.J Biomed Biotechnol. 2011;2011:928326. doi: 10.1155/2011/928326. Epub 2010 Nov 29. J Biomed Biotechnol. 2011. PMID: 21151616 Free PMC article. Review.
-
HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes.Epigenetics. 2015;10(5):418-30. doi: 10.1080/15592294.2015.1024406. Epub 2015 May 5. Epigenetics. 2015. PMID: 25941940 Free PMC article.
-
Emodin and emodin-rich rhubarb inhibits histone deacetylase (HDAC) activity and cardiac myocyte hypertrophy.J Nutr Biochem. 2020 May;79:108339. doi: 10.1016/j.jnutbio.2019.108339. Epub 2020 Jan 10. J Nutr Biochem. 2020. PMID: 32007664 Free PMC article.
-
[The mechanism underlying histone deacetylases regulating cardiac hypertrophy].Yi Chuan. 2020 Jun 20;42(6):536-547. doi: 10.16288/j.yczz.19-346. Yi Chuan. 2020. PMID: 32694112 Review. Chinese.
-
Histone deacetylases in cardiac fibrosis: current perspectives for therapy.Cell Signal. 2014 Mar;26(3):521-7. doi: 10.1016/j.cellsig.2013.11.037. Epub 2013 Dec 7. Cell Signal. 2014. PMID: 24321371 Review.
Cited by
-
Class I and II Histone Deacetylase Inhibitors as Therapeutic Modulators of Dilated Cardiac Tissue-Derived Mesenchymal Stem/Stromal Cells.Int J Mol Sci. 2024 Jun 19;25(12):6758. doi: 10.3390/ijms25126758. Int J Mol Sci. 2024. PMID: 38928463 Free PMC article.
-
Multi-omics association study of DNA methylation and gene expression levels and diagnoses of cardiovascular diseases in Danish Twins.Clin Epigenetics. 2024 Aug 26;16(1):117. doi: 10.1186/s13148-024-01727-6. Clin Epigenetics. 2024. PMID: 39187864 Free PMC article.
-
Histone deacetylase 6 as a novel promising target to treat cardiovascular disease.Cancer Innov. 2024 May 7;3(3):e114. doi: 10.1002/cai2.114. eCollection 2024 Jun. Cancer Innov. 2024. PMID: 38947757 Free PMC article. Review.
-
The Microenvironment of the Pathogenesis of Cardiac Hypertrophy.Cells. 2023 Jul 4;12(13):1780. doi: 10.3390/cells12131780. Cells. 2023. PMID: 37443814 Free PMC article. Review.
-
Drug repurposing for renin inhibition: identifying panobinostat for hypertension management.Mol Divers. 2025 Jun 23. doi: 10.1007/s11030-025-11253-z. Online ahead of print. Mol Divers. 2025. PMID: 40549296
References
Publication types
LinkOut - more resources
Full Text Sources

