UBE2L3 promotes lung adenocarcinoma invasion and metastasis through the GSK-3β/Snail signaling pathway
- PMID: 35958458
- PMCID: PMC9360901
UBE2L3 promotes lung adenocarcinoma invasion and metastasis through the GSK-3β/Snail signaling pathway
Abstract
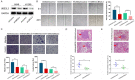
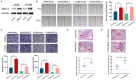
Lung cancer is the leading cause of cancer-related mortality, and the deaths are mostly attributed to distant metastasis. Previous studies have demonstrated that ubiquitin-conjugating enzyme E2 L3 (UBE2L3) mediates the progression of many human cancers. However, the roles and molecular mechanisms of UBE2L3 in invasion and metastasis of lung adenocarcinoma (LUAD) are yet to be fully understood. Here, we studied the expression pattern of UBE2L3 and demonstrated that it is dramatically up-regulated in LUAD tissues compared with the normal tissues, and its overexpression is positively correlated with lymph node metastasis. Moreover, the upregulation of UBEE2L3 in LUAD tissues is associated with shorter overall survival (OS). UBE2L3 silencing impairs the metastatic capacity of LUAD cells in vitro and in vivo, while its overexpression confers an opposite effect. In addition, our data showed that UBE2L3 promotes cancer cells epithelial-mesenchymal transition (EMT) and metastasis via the glycogen synthase kinase 3β (GSK-3β)/Snail axis. Besides, UBE2L3 was shown to promote ubiquitination and degradation of the GSK-3β. Immunohistochemical analysis demonstrated that UBE2L3 expression is positively correlated with Snail, but negatively correlated with GSK-3β and E-cadherin in LUAD tissues. Taken together, our findings demonstrated that UBE2L3 modulates metastasis of LUAD cells.
Keywords: EMT; GSK-3β; Lung adenocarcinoma; Snail; metastasis.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin. 2020;13:17–33. - PubMed
-
- Melosky B, Wheatley-Price P, Juergens RA, Sacher A, Leighl NB, Tsao MS, Cheema P, Snow S, Liu G, Card PB, Chu Q. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer. 2021;160:136–151. - PubMed
-
- Simeone JC, Nordstrom BL, Patel K, Klein AB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, US setting. Future Oncol. 2019;15:3491–3502. - PubMed
-
- Tan Q, Cui J, Huang J, Ding ZP, Lin H, Niu XM, Li ZM, Wang G, Luo QQ, Lu S. Genomic alteration during metastasis of lung adenocarcinoma. Cell Physiol Biochem. 2016;38:469–486. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
