Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer
- PMID: 35958515
- PMCID: PMC9362997
- DOI: 10.1093/rb/rbac051
Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer
Abstract
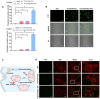
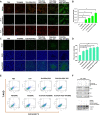
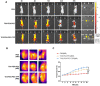
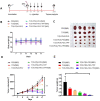
Targeting mitochondria via nano platform emerged as an attractive anti-tumor pathway due to the central regulation role in cellar apoptosis and drug resistance. Here, a mitochondria-targeting nanoparticle (TOS-PDA-PEG-TPP) was designed to precisely deliver polydopamine (PDA) as the photothermal agent and alpha-tocopherol succinate (α-TOS) as the chemotherapeutic drug to the mitochondria of the tumor cells, which inhibits the tumor growth through chemo- and photothermal- synergistic therapies. TOS-PDA-PEG-TPP was constructed by coating PDA on the surface of TOS NPs self-assembled by α-TOS, followed by grafting PEG and triphenylphosphonium (TPP) on their surface to prolong the blood circulation time and target delivery of TOS and PDA to the mitochondria of tumor cells. In vitro studies showed that TOS-PDA-PEG-TPP could be efficiently internalized by tumor cells and accumulated at mitochondria, resulting in cellular apoptosis and synergistic inhibition of tumor cell proliferation. In vivo studies demonstrated that TOS-PDA-PEG-TPP could be efficiently localized at tumor sites and significantly restrain the tumor growth under NIR irradiation without apparent toxicity or deleterious effects. Conclusively, the combination strategy adopted for functional nanodrugs construction aimed at target-delivering therapeutic agents with different action mechanisms to the same intracellular organelles can be extended to other nanodrugs-dependent therapeutic systems.
Keywords: alpha-tocopherol succinate (α-TOS); chemo- and photothermal- synergistic therapies; lung cancer; mitochondria-targeting.
© The Author(s) 2022. Published by Oxford University Press.
Figures



Similar articles
-
Mitochondria-Targeting Polydopamine Nanoparticles To Deliver Doxorubicin for Overcoming Drug Resistance.ACS Appl Mater Interfaces. 2017 May 24;9(20):16793-16802. doi: 10.1021/acsami.7b01540. Epub 2017 May 10. ACS Appl Mater Interfaces. 2017. PMID: 28481505
-
Synergistic antitumor efficacy of hybrid micelles with mitochondrial targeting and stimuli-responsive drug release behavior.J Mater Chem B. 2019 Mar 7;7(9):1415-1426. doi: 10.1039/c8tb02843e. Epub 2019 Feb 4. J Mater Chem B. 2019. PMID: 32255012
-
Polydopamine Nanoparticles Camouflaged by Stem Cell Membranes for Synergistic Chemo-Photothermal Therapy of Malignant Bone Tumors.Int J Nanomedicine. 2020 Dec 14;15:10183-10197. doi: 10.2147/IJN.S282931. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33363374 Free PMC article.
-
Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment.Macromol Biosci. 2020 Oct;20(10):e2000222. doi: 10.1002/mabi.202000222. Epub 2020 Aug 6. Macromol Biosci. 2020. PMID: 32761887 Review.
-
The role of alpha tocopheryl succinate (α-TOS) as a potential anticancer agent.Nutr Cancer. 2014;66(2):167-76. doi: 10.1080/01635581.2014.863367. Epub 2013 Dec 23. Nutr Cancer. 2014. PMID: 24364743 Review.
Cited by
-
Self-assembled albumin nanoparticles induce pyroptosis for photodynamic/photothermal/immuno synergistic therapies in triple-negative breast cancer.Front Immunol. 2023 May 26;14:1173487. doi: 10.3389/fimmu.2023.1173487. eCollection 2023. Front Immunol. 2023. PMID: 37342347 Free PMC article.
-
Regulation of mitochondrial network architecture and function in mesenchymal stem cells by micropatterned surfaces.Regen Biomater. 2024 May 7;11:rbae052. doi: 10.1093/rb/rbae052. eCollection 2024. Regen Biomater. 2024. PMID: 38854681 Free PMC article.
-
[Application of Nano-drug Delivery Technology in Overcoming Drug Resistance in Lung Cancer].Zhongguo Fei Ai Za Zhi. 2024 Nov 20;27(11):864-872. doi: 10.3779/j.issn.1009-3419.2024.101.30. Zhongguo Fei Ai Za Zhi. 2024. PMID: 39800482 Free PMC article. Review. Chinese.
-
Cancer cell response to extrinsic and intrinsic mechanical cue: opportunities for tumor apoptosis strategies.Regen Biomater. 2024 Feb 20;11:rbae016. doi: 10.1093/rb/rbae016. eCollection 2024. Regen Biomater. 2024. PMID: 38476678 Free PMC article. Review.
-
Doxorubicin-Loaded Fungal-Carboxymethyl Chitosan Functionalized Polydopamine Nanoparticles for Photothermal Cancer Therapy.Pharmaceutics. 2023 Apr 19;15(4):1281. doi: 10.3390/pharmaceutics15041281. Pharmaceutics. 2023. PMID: 37111766 Free PMC article.
References
-
- Chen F, An P, Liu L, Gao Z, Li Y, Zhang Y, Sun B, Zhou J.. A polydopamine-gated biodegradable cascade nanoreactor for pH-triggered and photothermal-enhanced tumor-specific nanocatalytic therapy. Nanoscale 2021;13:15677–88. - PubMed
-
- Dai G, Chu JCH, Chan CKW, Choi CHJ, Ng DKP.. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021;13:15899–915. - PubMed
-
- Fan B, Fan Q, Hu L, Cui M, Wang X, Ma H, Wei Q.. Polydopamine-PEG-folic acid conjugate film engineered TiO2 nanotube arrays for photoelectrochemical sensing of folate binding protein. ACS Appl Mater Interfaces 2020;12:1877–84. - PubMed
-
- Fang Y, Xing C, Zhan S, Zhao M, Li M, Liu H, Wang C.. Multifunctional magnetic-fluorescent nanoparticle: fabrication, bioimaging, and potential antibacterial applications. ACS Biomater Sci Eng 2019;5:6779–93. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

