Research and clinical translation of trilayer stent-graft of expanded polytetrafluoroethylene for interventional treatment of aortic dissection
- PMID: 35958517
- PMCID: PMC9362767
- DOI: 10.1093/rb/rbac049
Research and clinical translation of trilayer stent-graft of expanded polytetrafluoroethylene for interventional treatment of aortic dissection
Abstract
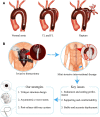
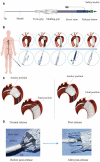
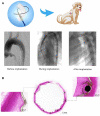
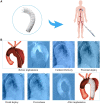
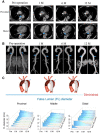
The aortic dissection (AD) is a life-threatening disease. The transcatheter endovascular aortic repair (EVAR) affords a minimally invasive technique to save the lives of these critical patients, and an appropriate stent-graft gets to be the key medical device during an EVAR procedure. Herein, we report a trilayer stent-graft and corresponding delivery system used for the treatment of the AD disease. The stent-graft is made of nitinol stents with an asymmetric Z-wave design and two expanded polytetrafluoroethylene (ePTFE) membranes. Each of the inner and outer surfaces of the stent-graft was covered by an ePTFE membrane, and the two membranes were then sintered together. The biological studies of the sintered ePTFE membranes indicated that the stent-graft had excellent cytocompatibility and hemocompatibility in vitro. Both the stent-graft and the delivery system exhibited satisfactory mechanical properties and operability. The safety and efficacy of this stent-graft and the corresponding delivery system were demonstrated in vivo. In nine canine experiments, the blood vessels of the animals implanted with the stent-grafts were of good patency, and there were no thrombus and obvious stenosis by angiography after implantation for 6 months. Furthermore, all of the nine clinical cases experienced successful implantation using the stent-graft and its postrelease delivery system, and the 1-year follow-ups indicated the preliminary safety and efficacy of the trilayer stent-graft with an asymmetric Z-wave design for interventional treatment.
Keywords: aortic dissection; clinical translation of biomaterials; delivery system for interventional treatment; expanded polytetrafluoroethylene; stent-graft.
© The Author(s) 2022. Published by Oxford University Press.
Figures




Similar articles
-
Comparison of the deployment and healing of thin-walled expanded PTFE stented grafts and covered stents.Ann Vasc Surg. 1996 Jul;10(4):336-46. doi: 10.1007/BF02286777. Ann Vasc Surg. 1996. PMID: 8879388
-
Healing response of normal canine aorta and iliac artery to a nitinol stent encapsulated in carbon-lined ePTFE.J Endovasc Ther. 2001 Jun;8(3):274-81. doi: 10.1177/152660280100800307. J Endovasc Ther. 2001. PMID: 11491262
-
Evaluation of pressure transmission and intra-aneurysmal contents after endovascular repair using the Trivascular Enovus expanded polytetrafluoroethylene stent graft in a canine model of abdominal aortic aneurysm.J Vasc Surg. 2007 Nov;46(5):1005-13. doi: 10.1016/j.jvs.2007.06.041. Epub 2007 Oct 1. J Vasc Surg. 2007. PMID: 17905556
-
Creation of transjugular intrahepatic portosystemic shunts with stent-grafts: initial experiences with a polytetrafluoroethylene-covered nitinol endoprosthesis.Radiology. 2001 Nov;221(2):437-46. doi: 10.1148/radiol.2212010195. Radiology. 2001. PMID: 11687688 Clinical Trial.
-
Endovascular exclusion of popliteal artery aneurysms with expanded polytetrafluoroethylene stent-grafts: early results.Vasc Endovascular Surg. 2006 Dec-2007 Jan;40(6):460-6. doi: 10.1177/1538574406294366. Vasc Endovascular Surg. 2006. PMID: 17202092
Cited by
-
Effects of serum proteins on corrosion rates and product bioabsorbability of biodegradable metals.Regen Biomater. 2023 Dec 12;11:rbad112. doi: 10.1093/rb/rbad112. eCollection 2024. Regen Biomater. 2023. PMID: 38173765 Free PMC article.
-
Biaxial stretching of polytetrafluoroethylene in industrial scale to fabricate medical ePTFE membrane with node-fibril microstructure.Regen Biomater. 2023 Jun 2;10:rbad056. doi: 10.1093/rb/rbad056. eCollection 2023. Regen Biomater. 2023. PMID: 37397871 Free PMC article.
-
Polyetheretherketone implants with hierarchical porous structure for boosted osseointegration.Biomater Res. 2023 Jun 27;27(1):61. doi: 10.1186/s40824-023-00407-5. Biomater Res. 2023. PMID: 37370127 Free PMC article.
-
Endocytosis-mediated healing: recombinant human collagen type III chain-induced wound healing for scar-free recovery.Regen Biomater. 2025 Jan 8;12:rbae149. doi: 10.1093/rb/rbae149. eCollection 2025. Regen Biomater. 2025. PMID: 40124986 Free PMC article.
-
Nanocomposites with Optimized Polytetrafluoroethylene Content as a Reinforcement Agent in PA12 and PLA for Material Extrusion Additive Manufacturing.Polymers (Basel). 2023 Jun 22;15(13):2786. doi: 10.3390/polym15132786. Polymers (Basel). 2023. PMID: 37447432 Free PMC article.
References
-
- White A, Broder J, Mando-Vandrick J, Wendell J, Crowe J.. Acute aortic emergencies—part 2 aortic dissections. Adv Emerg Nurs J 2013;35:28–52. - PubMed
-
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo RRB, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, Manolis A, Meijboom F, Nienaber C, Roffi M, Rousseau H, Sechtem U, Sirnes P, Von Allmen R, Vrints C.. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873–926. - PubMed
-
- Ding H, Luo S, Liu Y, Huang W, Jiang M, Li J, Xie N, Fan X, Fan R, Luo J.. Outcomes of hybrid procedure for type B aortic dissection with an aberrant right subclavian artery. J Vasc Surg 2018;67:704–11. - PubMed
LinkOut - more resources
Full Text Sources

