Role of human metapneumovirus glycoprotein G in modulation of immune responses
- PMID: 35958551
- PMCID: PMC9357950
- DOI: 10.3389/fimmu.2022.962925
Role of human metapneumovirus glycoprotein G in modulation of immune responses
Abstract
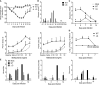
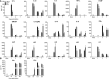
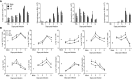
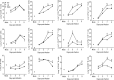
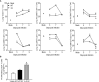
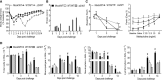
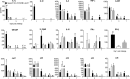
Human metapneumovirus (hMPV) is an important pathogen responsible for acute respiratory tract infections in children, the elderly, and immunocompromised patients, with no effective treatment or vaccine currently available. Knowledge of virus- and host-specific mechanisms contributing to the pathogenesis of hMPV infection is still limited. Studies have shown that hMPV surface glycoprotein G is an important virulence factor, by inhibiting innate immune signaling in airway epithelial cells and immune cells. In this study, we investigated the role of G protein in modulating innate and adaptive immune responses in mice infected with a recombinant virus with deletion of G protein (rhMPV-ΔG). Results show that rhMPV-ΔG was strongly attenuated, as it did not induce significant clinical disease, airway obstruction and airway hyperresponsiveness (AHR), compared to infection with a control strain (rhMPV-WT). By analysis of cells in bronchoalveolar fluid and lung tissue, as well as cytokine production, we found that G protein mediates aspects of both innate and adaptive immune responses, including neutrophils, dendritic cells, natural killer cells and B cells. Lung T cells recruited in response to rhMPV-ΔG had a significantly higher activated phenotype compared to those present after rhMPV-WT infection. Despite highly attenuation characterized by low levels of replication in the lung, rhMPV-ΔG was able to induce neutralizing antibodies and to protect mice from a secondary hMPV challenge. However, challenged mice that had received rhMPV-ΔG as primary infection showed some signs of lung disease at the earliest time points, which were less evident in mice that had received the rhMPV-WT strain as primary infection. These results demonstrate some of the mechanisms by which G protein could contribute to airway disease and modulate immune response to hMPV infection.
Keywords: RNA virus; hMPV G protein; immune response; lung disease; mouse model.
Copyright © 2022 Velayutham, Ivanciuc, Garofalo and Casola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Human metapneumovirus glycoprotein G inhibits innate immune responses.PLoS Pathog. 2008 May 30;4(5):e1000077. doi: 10.1371/journal.ppat.1000077. PLoS Pathog. 2008. PMID: 18516301 Free PMC article.
-
Human metapneumovirus glycoprotein G disrupts mitochondrial signaling in airway epithelial cells.PLoS One. 2013 Apr 23;8(4):e62568. doi: 10.1371/journal.pone.0062568. Print 2013. PLoS One. 2013. PMID: 23626834 Free PMC article.
-
Experimental infection of adults with recombinant wild-type human metapneumovirus.J Infect Dis. 2013 Nov 15;208(10):1669-78. doi: 10.1093/infdis/jit356. Epub 2013 Aug 1. J Infect Dis. 2013. PMID: 23908489 Free PMC article. Clinical Trial.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
-
Host Components That Modulate the Disease Caused by hMPV.Viruses. 2021 Mar 22;13(3):519. doi: 10.3390/v13030519. Viruses. 2021. PMID: 33809875 Free PMC article. Review.
Cited by
-
hMPV Outbreaks: Worldwide Implications of a Re-Emerging Respiratory Pathogen.Microorganisms. 2025 Jun 27;13(7):1508. doi: 10.3390/microorganisms13071508. Microorganisms. 2025. PMID: 40732017 Free PMC article. Review.
-
Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice.NPJ Vaccines. 2024 Jun 19;9(1):111. doi: 10.1038/s41541-024-00899-9. NPJ Vaccines. 2024. PMID: 38898106 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

