Early detection of soluble CD27, BTLA, and TIM-3 predicts the development of nosocomial infection in pediatric burn patients
- PMID: 35958579
- PMCID: PMC9360547
- DOI: 10.3389/fimmu.2022.940835
Early detection of soluble CD27, BTLA, and TIM-3 predicts the development of nosocomial infection in pediatric burn patients
Abstract
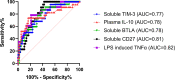
Thermal injury induces concurrent inflammatory and immune dysfunction, which is associated with adverse clinical outcomes. However, these effects in the pediatric population are less studied and there is no standard method to identify those at risk for developing infections. Our goal was to better understand immune dysfunction and identify soluble protein markers following pediatric thermal injury. Further we wanted to determine which early inflammatory, soluble, or immune function markers are most predictive of the development of nosocomial infections (NI) after burn injury. We performed a prospective observational study at a single American Burn Association-verified Pediatric Burn Center. A total of 94 pediatric burn subjects were enrolled and twenty-three of those subjects developed a NI with a median time to diagnosis of 8 days. Whole blood samples, collected within the first 72 hours after injury, were used to compare various markers of inflammation, immune function, and soluble proteins between those who recovered without developing an infection and those who developed a NI after burn injury. Within the first three days of burn injury, innate and adaptive immune function markers (ex vivo lipopolysaccharide-induced tumor necrosis factor alpha production capacity, and ex vivo phytohemagglutinin-induced interleukin-10 production capacity, respectively) were decreased for those subjects who developed a subsequent NI. Further analysis of soluble protein targets associated with these pathways displayed significant increases in soluble CD27, BTLA, and TIM-3 for those who developed a NI. Our findings indicate that suppression of both the innate and adaptive immune function occurs concurrently within the first 72 hours following pediatric thermal injury. At the same time, subjects who developed NI have increased soluble protein biomarkers. Soluble CD27, BTLA, and TIM-3 were highly predictive of the development of subsequent infectious complications. This study identifies early soluble protein makers that are predictive of infection in pediatric burn subjects. These findings should inform future immunomodulatory therapeutic studies.
Keywords: immune checkpoint inhibitors; immune dysfunction; inflammation; nosocomial infection; pediatric thermal injury; soluble proteins.
Copyright © 2022 Penatzer, Alexander, Simon, Wolfe, Breuer, Hensley, Fabia, Hall and Thakkar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Measures of Adaptive Immune Function Predict the Risk of Nosocomial Infection in Pediatric Burn Patients.J Burn Care Res. 2022 Nov 2;43(6):1416-1425. doi: 10.1093/jbcr/irac050. J Burn Care Res. 2022. PMID: 35436346 Free PMC article.
-
Reversibility of Immune Dysfunction Following Pediatric Thermal Injury.J Burn Care Res. 2025 Aug 1:iraf152. doi: 10.1093/jbcr/iraf152. Online ahead of print. J Burn Care Res. 2025. PMID: 40747976
-
Measures of Systemic Innate Immune Function Predict the Risk of Nosocomial Infection in Pediatric Burn Patients.J Burn Care Res. 2021 May 7;42(3):488-494. doi: 10.1093/jbcr/iraa193. J Burn Care Res. 2021. PMID: 33128368 Free PMC article.
-
The systemic immune response to pediatric thermal injury.Int J Burns Trauma. 2018 Feb 5;8(1):6-16. eCollection 2018. Int J Burns Trauma. 2018. PMID: 29531854 Free PMC article. Review.
-
Biomarkers of sepsis in burn injury: an update.Burns Trauma. 2025 Jan 16;13:tkae080. doi: 10.1093/burnst/tkae080. eCollection 2025. Burns Trauma. 2025. PMID: 39822649 Free PMC article. Review.
Cited by
-
BTLA biology in cancer: from bench discoveries to clinical potentials.Biomark Res. 2024 Jan 17;12(1):8. doi: 10.1186/s40364-024-00556-2. Biomark Res. 2024. PMID: 38233898 Free PMC article. Review.
-
Pneumonia in the first week after polytrauma is associated with reduced blood levels of soluble herpes virus entry mediator.Front Immunol. 2023 Dec 22;14:1259423. doi: 10.3389/fimmu.2023.1259423. eCollection 2023. Front Immunol. 2023. PMID: 38187375 Free PMC article.
-
Predicting survival in patients with SARS-CoV-2 based on cytokines and soluble immune checkpoint regulators.Front Cell Infect Microbiol. 2024 Nov 25;14:1397297. doi: 10.3389/fcimb.2024.1397297. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39654974 Free PMC article.
-
FAS(APO), DAMP, and AKT Phosphoproteins Expression Predict the Development of Nosocomial Infection After Pediatric Burn Injury.J Burn Care Res. 2024 Nov 14;45(6):1607-1616. doi: 10.1093/jbcr/irae111. J Burn Care Res. 2024. PMID: 38863248 Free PMC article.
References
-
- American Burn Association . National burn repository 2019 update: Report of data from 2009-2018. (2019). Available at: https://sk75w2kudjd3fv2xs2cvymrg-wpengine.netdna-ssl.com/wp-content/uplo....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

