Immunogenicity and safety of concomitant administration of the chinese inactivated poliovirus vaccine with the diphtheria-tetanus-acellular pertussis (DTaP) vaccine in children: A multicenter, randomized, non-inferiority, controlled trial
- PMID: 35958596
- PMCID: PMC9361845
- DOI: 10.3389/fimmu.2022.905634
Immunogenicity and safety of concomitant administration of the chinese inactivated poliovirus vaccine with the diphtheria-tetanus-acellular pertussis (DTaP) vaccine in children: A multicenter, randomized, non-inferiority, controlled trial
Abstract
Key point: Considering that vaccination with the sIPV and DTaP overlap at the ages of 3 and 4 months in China, to reduce the burden of treatment on parents and increase vaccination coverage rates, we designed a postmarket clinical study of co-administration.
Background: The Sabin-strain-based inactivated poliovirus vaccine (sIPV) and the diphtheria-tetanus-acellular pertussis vaccine (DTaP) have been licensed in China for many years. To conduct a clinical study on the safety and immunogenicity of the sIPV when administered concomitantly with the DTaP.
Methods: The study population was divided into three groups: group 1 was the sIPV+ DTaP concomitant administration group, group 2 was the sIPV inoculation group, and group 3 was the DTaP inoculation group. Blood samples were collected prevaccination and 30 days postvaccination, and serum antibody levels were detected.
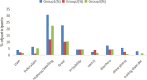
Results: This study showed that the seropositive and seroconversion rates of type 1, 2 and 3 poliovirus in group 1 were higher than those in group 2, with no statistically significant difference after vaccination (P>0.05). Groups 1 and 3 also showed similar responses for all vaccine antigens except anti-FHA (97.65 (94.09-99.36) vs. 100 (97.89-100)). The geometric mean titers (GMTs) for the DTaP and sIPV among the groups were comparable, and the non-inferiority t test result was P<0.001. The number of local adverse events (AEs) reported in group 1 (29.91%) were larger than those in group 2 (12.39%) and group 3 (21.93%), among which the most common was redness. Similarly, the most common systemic AE was fever. All 5 severe AE (SAE) cases were determined by experts to be unrelated to the vaccines during the study.
Conclusions: The evidence of similar seroconversion and safety with co-administered DTaP and sIPV supports the co-administration supports the introduction of a strategy of simultaneous administration of both vaccines into routine infant immunization, and it could increase vaccination coverage and protect more infants from morbidity and mortality from these related diseases.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT04054882?term=NCT04054882&cntry=CN&draw=2&rank=1, identifier NCT04054882.
Keywords: DTaP; concomitant administration; immunogenicity; sIPV; safety; vaccine interference.
Copyright © 2022 Sun, Xu, Tang, Xiao, Wang, Wang, Zhu, Yang and Chen.
Conflict of interest statement
YH X, HC and XY are employees of China National Biotec Group Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

 , blood sample;
, blood sample; , sIPV;
, sIPV; , DTaP.).
, DTaP.).

References
-
- Sobanjo-Ter MA, Duclos P, McIntyre P, Lewis KD, Van Damme P, O'Brien KL, et al. Assessing the evidence for maternal pertussis immunization: a report from the bill & melinda gates foundation symposium on pertussis infant disease burden in low- and lower-middle-income countries. Clin Infect Dis (2016) 63:S123–33. doi: 10.1093/cid/ciw530 - DOI - PMC - PubMed
-
- Yan S, Chen H, Zhang Z, Chang S, Xiao Y, Luo L, et al. Immunogenicity and safety of different sequential schedules of Sabin strain-based inactivated poliovirus vaccination: A randomized, controlled, open-label, phase IV clinical trial in China. VACCINE (2020) 38:6274–9. doi: 10.1016/j.vaccine.2020.07.042 - DOI - PubMed
-
- Zhao YS. Analysis on the deveiopment trend and problems of combined vaccine industry in China. Chin J Pharm (2018) 49:1306–12. SQ WLEA. doi: 10.16522/j.cnki.cjph.2018.09.016 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

