Efficacy and Safety of Neoadjuvant Monoimmunotherapy With PD-1 Inhibitor for dMMR/MSI⁃H Locally Advanced Colorectal Cancer: A Single-Center Real-World Study
- PMID: 35958603
- PMCID: PMC9359076
- DOI: 10.3389/fimmu.2022.913483
Efficacy and Safety of Neoadjuvant Monoimmunotherapy With PD-1 Inhibitor for dMMR/MSI⁃H Locally Advanced Colorectal Cancer: A Single-Center Real-World Study
Abstract
Objective: To explore the efficacy and safety of single-agent programmed cell death protein-1 (PD-1) inhibitor in the neoadjuvant treatment of patients with mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) locally advanced colorectal cancer (LACRC) through single-center large⁃sample analysis based on real⁃world data in China.
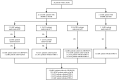
Methods: This study was a retrospective, single-center, case series study. 33 colorectal cancer (CRC) patients with clinical stage of T3~4N0~2M0 treated in Yunnan Cancer Hospital from June 2019 to June 2021 were analyzed retrospectively. Among them, 32 patients were dMMR or MSI-H or both dMMR and MSI-H, and one patient was both dMMR and microsatellite stability (MSS) (excluded in the final analysis). All 32 patients received neoadjuvant immunotherapy (nIT) with single-agent PD⁃1 inhibitor.
Results: Among the 32 patients, 8 (25%) were locally advanced rectal cancer (LARC) and 24 (75%) were locally advanced colon cancer (LACC); 4 (12.55%) were stage II and 28 (87.5%) were stage III. The median number of cycles of 32 patients with dMMR/MSI-H LACRC receiving nIT with single-agent PD-1 blockade was 6 (4~10), and the median number of cycles to achieve partial response (PR) was 3 (2~4). Among them, three LARC patients achieved clinical complete response (cCR) and adopted the watch-and-wait (W&W) strategy. The objective response rate (ORR) of the other 29 patients with radical surgery was 100% (29/29), the pathological response rate was 100% (29/29), the rate of major pathological response (MPR) was 86.2% (25/29), and the rate of pathological complete response (pCR) was 75.9% (22/29). The incidence of immune-related adverse events (irAEs) in 32 patients during nIT was 37.5% (12/32), while the incidence of irAEs in 22 patients with operation during adjuvant immunotherapy was 27.3% (6/22), all of which were grade 1~2. No grade 3 or above irAEs were occured. The median time from the last nIT to surgery was 27 (16~42) days. There were no delayed radical resection due to irAEs in these patients. All 29 patients achieved R0 resection. The incidence of surgical-related adverse events (srAEs) in perioperative period was 10.3% (3/29).
Conclusions: Neoadjuvant monoimmunotherapy with PD-1 inhibitor has favorable ORR and pCR rate, and relatively low incidences of irAEs and srAEs for patients with dMMR/MSI-H LACRC, suggesting that this nIT regimen of single-agent PD-1 inhibitor is significantly effective and sufficiently safe.
Keywords: locally advanced colorectal cancer; microsatellite instability-high (MSI-H); mismatch repair-deficient; neoadjuvant immunotherapy; programmed cell death protein-1 inhibitor.
Copyright © 2022 Zhang, Yang, Wu, Cai, Li, Yu, Li, Ding, Dong, Li, Hu, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study.Front Immunol. 2023 Jun 27;14:1182299. doi: 10.3389/fimmu.2023.1182299. eCollection 2023. Front Immunol. 2023. PMID: 37441082 Free PMC article.
-
Single-Agent Neoadjuvant Immunotherapy With a PD-1 Antibody in Locally Advanced Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer.Clin Colorectal Cancer. 2023 Mar;22(1):85-91. doi: 10.1016/j.clcc.2022.11.004. Epub 2022 Nov 25. Clin Colorectal Cancer. 2023. PMID: 36528470
-
[Multicenter real-world study on safety and efficacy of neoadjuvant therapy in combination with immunotherapy for colorectal cancer].Zhonghua Wei Chang Wai Ke Za Zhi. 2022 Mar 25;25(3):219-227. doi: 10.3760/cma.j.cn441530-20220228-00070. Zhonghua Wei Chang Wai Ke Za Zhi. 2022. PMID: 35340171 Chinese.
-
Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities.Front Immunol. 2022 Mar 17;13:795972. doi: 10.3389/fimmu.2022.795972. eCollection 2022. Front Immunol. 2022. PMID: 35371084 Free PMC article. Review.
-
Neoadjuvant immunotherapy for DNA mismatch repair proficient/microsatellite stable non-metastatic rectal cancer: a systematic review and meta-analysis.Front Immunol. 2025 Jan 27;16:1523455. doi: 10.3389/fimmu.2025.1523455. eCollection 2025. Front Immunol. 2025. PMID: 39931055 Free PMC article.
Cited by
-
Perioperative immune checkpoint inhibition for colorectal cancer: recent advances and future directions.Front Immunol. 2023 Nov 13;14:1269341. doi: 10.3389/fimmu.2023.1269341. eCollection 2023. Front Immunol. 2023. PMID: 38022667 Free PMC article. Review.
-
Neoadjuvant immunotherapy for dMMR and pMMR colorectal cancers: therapeutic strategies and putative biomarkers of response.Nat Rev Clin Oncol. 2024 Dec;21(12):839-851. doi: 10.1038/s41571-024-00943-6. Epub 2024 Sep 24. Nat Rev Clin Oncol. 2024. PMID: 39317818 Review.
-
The neoadjuvant immunotherapy for non-metastatic mismatch repair-deficient colorectal cancer: a systematic review.Front Immunol. 2025 May 1;16:1540751. doi: 10.3389/fimmu.2025.1540751. eCollection 2025. Front Immunol. 2025. PMID: 40376001 Free PMC article.
-
Therapeutic targeting of mismatch repair-deficient cancers.Nat Rev Clin Oncol. 2025 Jul 10. doi: 10.1038/s41571-025-01054-6. Online ahead of print. Nat Rev Clin Oncol. 2025. PMID: 40640471 Review.
-
T cell factor 1 (TCF-1) defines T cell differentiation in colorectal cancer.iScience. 2024 Aug 22;27(9):110754. doi: 10.1016/j.isci.2024.110754. eCollection 2024 Sep 20. iScience. 2024. PMID: 39280606 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

