Adoptive B cell therapy for chronic viral infection
- PMID: 35958615
- PMCID: PMC9361846
- DOI: 10.3389/fimmu.2022.908707
Adoptive B cell therapy for chronic viral infection
Abstract
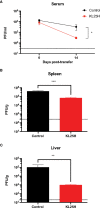
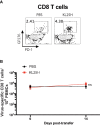
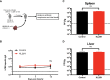
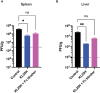
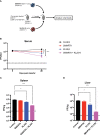
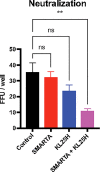
T cell-based therapies have been widely explored for the treatment of cancer and chronic infection, but B cell-based therapies have remained largely unexplored. To study the effect of B cell therapy, we adoptively transferred virus-specific B cells into mice that were chronically infected with lymphocytic choriomeningitis virus (LCMV). Adoptive transfer of virus-specific B cells resulted in increase in antibody titers and reduction of viral loads. Importantly, the efficacy of B cell therapy was partly dependent on antibody effector functions, and was improved by co-transferring virus-specific CD4 T cells. These findings provide a proof-of-concept that adoptive B cell therapy can be effective for the treatment of chronic infections, but provision of virus-specific CD4 T cells may be critical for optimal virus neutralization.
Keywords: B cells; adoptive cell therapy; chronic viral infection; lymphocytic choriomeningitis virus (LCMV); virus.
Copyright © 2022 Chung, Dangi, Palacio, Sanchez and Penaloza-MacMaster.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers.Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6874-9. doi: 10.1073/pnas.94.13.6874. Proc Natl Acad Sci U S A. 1997. PMID: 9192659 Free PMC article.
-
Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice.J Virol. 1998 Nov;72(11):9208-16. doi: 10.1128/JVI.72.11.9208-9216.1998. J Virol. 1998. PMID: 9765468 Free PMC article.
-
Bone marrow contains virus-specific cytotoxic T lymphocytes.Blood. 1997 Sep 1;90(5):2103-8. Blood. 1997. PMID: 9292550
-
Loss of Resistance to Mousepox during Chronic Lymphocytic Choriomeningitis Virus Infection Is Associated with Impaired T-Cell Responses and Can Be Rescued by Immunization.J Virol. 2020 Feb 14;94(5):e01832-19. doi: 10.1128/JVI.01832-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31826990 Free PMC article.
-
Exhaustion of cytotoxic T cells during adoptive immunotherapy of virus carrier mice can be prevented by B cells or CD4+ T cells.Eur J Immunol. 2002 Feb;32(2):374-82. doi: 10.1002/1521-4141(200202)32:2<374::AID-IMMU374>3.0.CO;2-9. Eur J Immunol. 2002. PMID: 11813156
Cited by
-
Meeting the Challenge of Controlling Viral Immunopathology.Int J Mol Sci. 2024 Apr 1;25(7):3935. doi: 10.3390/ijms25073935. Int J Mol Sci. 2024. PMID: 38612744 Free PMC article. Review.
-
Effects of protein boosters on antibody responses.bioRxiv [Preprint]. 2025 Feb 11:2025.02.08.637239. doi: 10.1101/2025.02.08.637239. bioRxiv. 2025. PMID: 39990358 Free PMC article. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

