Right Heart Failure in Mice Upon Pressure Overload Is Promoted by Mitochondrial Oxidative Stress
- PMID: 35958691
- PMCID: PMC9357563
- DOI: 10.1016/j.jacbts.2022.02.018
Right Heart Failure in Mice Upon Pressure Overload Is Promoted by Mitochondrial Oxidative Stress
Abstract
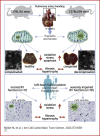
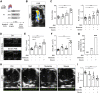
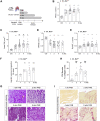
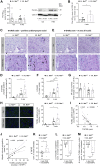
We sought to unravel pathomechanisms of the transition of maladaptive right ventricular (RV) remodeling to right heart failure (RHF) upon pressure overload. Exposure of C57BL/6J and C57BL/6N mice to pulmonary artery banding disclosed a tight relation of structural remodeling with afterload, but a dissociation from RV systolic function. Reduced release of mitochondrial reactive oxygen species in C57BL/6J mice prevented the development of RHF. In patients with left heart failure, increased oxidative damage in RV sections was associated with severely impaired RV function. In conclusion, reactive oxygen species are involved in the transition of maladaptive RV remodeling to RHF.
Keywords: 6J, C57BL/6J; 6N, C57BL/6N; NNT, nicotinamide nucleotide transhydrogenase; PAB, pulmonary artery banding; RHF, right heart failure; RVD, right ventricular dysfunction; RVH, right ventricular hypertrophy; TAPSE, tricuspid annular plane systolic excursion; iRVF, severely impaired right ventricular function; nRVF, normal right ventricular function; oxidative stress; pressure overload; pulmonary artery banding; reactive oxygen species; right heart failure.
© 2022 The Authors.
Conflict of interest statement
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn, Germany (RU 1678/3-3 to Dr Rudolph), by the Deutsche Stiftung für Herzforschung, Frankfurt a.M., Germany (F/ 48/ 20 to Drs Müller and Klinke) and by FoRUM, Bochum, Germany (F991R-21 to Drs Rudolph and Klinke). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




Similar articles
-
Extracellular matrix instability and chronic inflammation underlie maladaptive right ventricular pressure overload remodeling and failure in male mice.Am J Physiol Heart Circ Physiol. 2025 Mar 1;328(3):H676-H692. doi: 10.1152/ajpheart.00331.2024. Epub 2024 Dec 16. Am J Physiol Heart Circ Physiol. 2025. PMID: 39679492
-
FHL-1 is not involved in pressure overload-induced maladaptive right ventricular remodeling and dysfunction.Basic Res Cardiol. 2020 Jan 24;115(2):17. doi: 10.1007/s00395-019-0767-5. Basic Res Cardiol. 2020. PMID: 31980934 Free PMC article.
-
Lack of right ventricular hypertrophy is associated with right heart failure in patients with left ventricular failure.Heart Vessels. 2022 Oct;37(10):1728-1739. doi: 10.1007/s00380-022-02075-2. Epub 2022 Apr 26. Heart Vessels. 2022. PMID: 35471461
-
The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure.Circ Res. 2014 Jun 20;115(1):176-88. doi: 10.1161/CIRCRESAHA.113.301129. Circ Res. 2014. PMID: 24951766 Free PMC article. Review.
-
The Dark Side of the Moon: The Right Ventricle.J Cardiovasc Dev Dis. 2017 Oct 20;4(4):18. doi: 10.3390/jcdd4040018. J Cardiovasc Dev Dis. 2017. PMID: 29367547 Free PMC article. Review.
Cited by
-
Mitochondrial Integrity Is Critical in Right Heart Failure Development.Int J Mol Sci. 2023 Jul 5;24(13):11108. doi: 10.3390/ijms241311108. Int J Mol Sci. 2023. PMID: 37446287 Free PMC article. Review.
-
Right ventricular failure in pulmonary hypertension: recent insights from experimental models.Herz. 2023 Aug;48(4):285-290. doi: 10.1007/s00059-023-05180-8. Epub 2023 Apr 20. Herz. 2023. PMID: 37079028 Review. English.
-
The unique hypertrophic and fibrotic features of neonatal right ventricle in response to pressure overload.Sci Rep. 2025 May 20;15(1):17525. doi: 10.1038/s41598-025-01427-y. Sci Rep. 2025. PMID: 40394014 Free PMC article.
-
Mechano-energetic uncoupling in heart failure.Nat Rev Cardiol. 2025 Jun 22. doi: 10.1038/s41569-025-01167-6. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40544170 Review.
-
Oxidative stress initiates hemodynamic change in CKD-induced heart disease.Basic Res Cardiol. 2024 Dec;119(6):957-971. doi: 10.1007/s00395-024-01085-7. Epub 2024 Oct 15. Basic Res Cardiol. 2024. PMID: 39404904 Free PMC article.
References
-
- Konstam M.A., Kiernan M.S., Bernstein D., et al. for the American Heart Association Council on Clinical Cardiology. Council on Cardiovascular Disease in the Young, and Council on Cardiovascular Surgery and Anesthesia Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. 2018;137:e578–e622. - PubMed
-
- Heitmeier T., Sydykov A., Lukas C., et al. Altered proteasome function in right ventricular hypertrophy. Cardiovasc Res. 2020;116:406–415. - PubMed
-
- Bogaard H.J., Natarajan R., Henderson S.C., et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120(20):1951–1960. - PubMed
LinkOut - more resources
Full Text Sources

