Two-dimensional correlated spectroscopy distinguishes clear cell renal cell carcinoma from other kidney neoplasms and non-cancer kidney
- PMID: 35958897
- PMCID: PMC9360516
- DOI: 10.21037/tau-21-1082
Two-dimensional correlated spectroscopy distinguishes clear cell renal cell carcinoma from other kidney neoplasms and non-cancer kidney
Abstract
Background: Routinely used clinical scanners, such as computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound (US), are unable to distinguish between aggressive and indolent tumor subtypes in masses localized to the kidney, often leading to surgical overtreatment. The results of the current investigation demonstrate that chemical differences, detected in human kidney biopsies using two-dimensional COrrelated SpectroscopY (2D L-COSY) and evaluated using multivariate statistical analysis, can distinguish these subtypes.
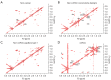
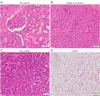
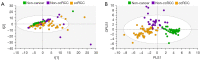
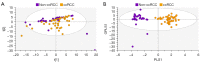
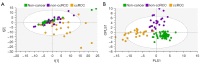
Methods: One hundred and twenty-six biopsy samples from patients with a confirmed enhancing kidney mass on abdominal imaging were analyzed as part of the training set. A further forty-three samples were used for model validation. In patients undergoing radical nephrectomy, biopsies of non-cancer kidney cortical tissue were also collected as a non-cancer control group. Spectroscopy data were analyzed using multivariate statistical analysis, including principal component analysis (PCA) and orthogonal projection to latent structures with discriminant analysis (OPLS-DA), to identify biomarkers in kidney cancer tissue that was also classified using the gold-standard of histopathology.
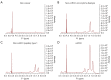
Results: The data analysis methodology showed good separation between clear cell renal cell carcinoma (ccRCC) versus non-clear cell RCC (non-ccRCC) and non-cancer cortical tissue from the kidneys of tumor-bearing patients. Variable Importance for the Projection (VIP) values, and OPLS-DA loadings plots were used to identify chemical species that correlated significantly with the histopathological classification. Model validation resulted in the correct classification of 37/43 biopsy samples, which included the correct classification of 15/17 ccRCC biopsies, achieving an overall predictive accuracy of 86%, Those chemical markers with a VIP value >1.2 were further analyzed using univariate statistical analysis. A subgroup analysis of 47 tumor tissues arising from T1 tumors revealed distinct separation between ccRCC and non-ccRCC tissues.
Conclusions: This study provides metabolic insights that could have future diagnostic and/or clinical value. The results of this work demonstrate a clear separation between clear cell and non-ccRCC and non-cancer kidney tissue from tumor-bearing patients. The clinical translation of these results will now require the development of a one-dimensional (1D) magnetic resonance spectroscopy (MRS) protocol, for the kidney, using an in vivo clinical MRI scanner.
Keywords: Renal cell carcinoma (RCC); magnetic resonance spectroscopy (MRS); multivariate statistical analysis; principal components analysis; spectroscopy-pathology correlation.
2022 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-21-1082/coif). SJDV received a University of Queensland Research Training Program Scholarship for her PhD studies and a Randal Silcock Bursary for conference travel. CEM has received research grants from Advance Queensland Research, was a consultant for the National Imaging Facility, and is a shareholder for DatChem Pty Ltd. and Goolwa Pty Ltd. The other authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
