CircAGFG1 Promotes Osteosarcoma Progression and Stemness by Competing with miR-302a-3p to Upregulate the Expression of LATS2
- PMID: 35958928
- PMCID: PMC9357677
- DOI: 10.1155/2022/6370766
CircAGFG1 Promotes Osteosarcoma Progression and Stemness by Competing with miR-302a-3p to Upregulate the Expression of LATS2
Retraction in
-
Retracted: CircAGFG1 Promotes Osteosarcoma Progression and Stemness by Competing with miR-302a-3p to Upregulate the Expression of LATS2.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9848914. doi: 10.1155/2023/9848914. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125174 Free PMC article.
Abstract
This study aimed to investigate the effect of circRNA (circAGFG1) on the proliferation, migration, invasion, and cell stemness of osteosarcoma cells by targeting miR-302a to regulate LATS2. The expression of circAGFG1 in osteosarcoma cells and normal osteoblasts was detected by real-time fluorescent quantitative PCR (RT-qPCR). Cell proliferation, clone formation, and invasion were detected by CCK-8, clone formation, and cell invasion assays. In vivo tumor formation assay was used to detect the effect of circAGFG1 on tumor growth. The expression level of circAGFG1 was upregulated in osteosarcoma cells. The downregulation of circAGFG1 inhibited the proliferation, invasion, and migration of osteosarcoma cells. The overexpression of circAGFG1 enhanced the stemness of osteosarcoma cells. CircAGFG1 was specifically bound to miR-302a to regulate the expression activity of miR-302a. MiR-302a specifically bound to the 3'UTR of LATS2 and inhibited the expression of LATS2. The overexpression of miR-302a reversed the effect of circAGFG1 on the proliferation, invasion, and migration of osteosarcoma cells. CircAGFG1 regulated the expression of LATS2 by miR-302a, thereby regulating the proliferation, migration, and invasion of osteosarcoma cells.
Copyright © 2022 Tongchun Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


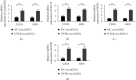

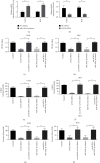

Similar articles
-
Retracted: CircAGFG1 Promotes Osteosarcoma Progression and Stemness by Competing with miR-302a-3p to Upregulate the Expression of LATS2.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9848914. doi: 10.1155/2023/9848914. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125174 Free PMC article.
-
MicroRNA-302a inhibits osteosarcoma cell migration and invasion by directly targeting IGF-1R.Oncol Lett. 2018 Apr;15(4):5577-5583. doi: 10.3892/ol.2018.8049. Epub 2018 Feb 14. Oncol Lett. 2018. Retraction in: Oncol Lett. 2022 Aug 26;24(4):360. doi: 10.3892/ol.2022.13480. PMID: 29563995 Free PMC article. Retracted.
-
miR-302a-3p suppresses melanoma cell progression via targeting METTL3.J Chemother. 2022 Feb;34(1):55-66. doi: 10.1080/1120009X.2021.1953886. Epub 2021 Jul 21. J Chemother. 2022. PMID: 34286671
-
CircAGFG1 promotes cervical cancer progression via miR-370-3p/RAF1 signaling.BMC Cancer. 2019 Nov 8;19(1):1067. doi: 10.1186/s12885-019-6269-x. BMC Cancer. 2019. PMID: 31703640 Free PMC article.
-
CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1.Cell Death Dis. 2020 Jul 17;11(7):542. doi: 10.1038/s41419-020-2707-6. Cell Death Dis. 2020. PMID: 32681092 Free PMC article.
Cited by
-
Circular RNAs in cancer stem cells: Insights into their roles and mechanisms (Review).Int J Mol Med. 2025 Mar;55(3):50. doi: 10.3892/ijmm.2025.5491. Epub 2025 Jan 24. Int J Mol Med. 2025. PMID: 39930823 Free PMC article. Review.
-
Retracted: CircAGFG1 Promotes Osteosarcoma Progression and Stemness by Competing with miR-302a-3p to Upregulate the Expression of LATS2.Evid Based Complement Alternat Med. 2023 Dec 13;2023:9848914. doi: 10.1155/2023/9848914. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 38125174 Free PMC article.
References
-
- Sayles L. C., Breese M. R., Koehne A. L., et al. Genome-informed targeted therapy for osteosarcoma. Cancer Discovery . 2019;9(1):46–63. doi: 10.1158/2159-8290.cd-17-1152. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

