Neurogenic Hypotension and Bradycardia Modulated by Electroacupuncture in Hypothalamic Paraventricular Nucleus
- PMID: 35958987
- PMCID: PMC9361000
- DOI: 10.3389/fnins.2022.934752
Neurogenic Hypotension and Bradycardia Modulated by Electroacupuncture in Hypothalamic Paraventricular Nucleus
Abstract
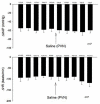
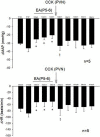
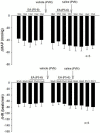
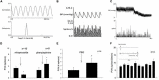
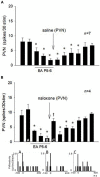
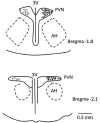
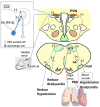
Electroacupuncture (EA) stimulates somatic median afferents underlying P5-6 acupoints and modulates parasympathoexcitatory reflex responses through central processing in the brainstem. Although decreases in blood pressure and heart rate by the neural-mediated Bezold-Jarisch reflex responses are modulated by EA through opioid actions in the nucleus tractus solitarius and nucleus ambiguus, the role of the hypothalamus is unclear. The hypothalamic paraventricular nucleus (PVN) is activated by sympathetic afferents and regulates sympathetic outflow and sympathoexcitatory cardiovascular responses. In addition, the PVN is activated by vagal afferents, but little is known about its regulation of cardiopulmonary inhibitory hemodynamic responses. We hypothesized that the PVN participates in the Bezold-Jarisch reflex responses and EA inhibits these cardiopulmonary responses through the PVN opioid system. Rats were anesthetized and ventilated, and their heart rate and blood pressures were monitored. Application of phenylbiguanide every 10 min close to the right atrium induced consistent depressor and bradycardia reflex responses. Unilateral microinjection of the depolarization blockade agent kainic acid or glutamate receptor antagonist kynurenic acid in the PVN reduced these reflex responses. In at least 70% of the rats, 30 min of bilateral EA at P5-6 acupoints reduced the depressor and bradycardia responses for at least 60 min. Blockade of the CCK-1 receptors converted the non-responders into EA-responders. Unilateral PVN-microinjection with naloxone reversed the EA inhibition. Vagal-evoked activity of the PVN cardiovascular neurons was reduced by 30 min EA (P5-6) through opioid receptor activation. These data indicate that PVN processes inhibitory cardiopulmonary reflexes and participates in EA-modulation of the neural-mediated vasodepression and bradycardia.
Keywords: CCK; opioids; parasympathetic; serotonin; somatosensory.
Copyright © 2022 Tjen-A-Looi, Fu, Guo, Gong, Nguyen, Nguyen and Malik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
GABA in nucleus tractus solitarius participates in electroacupuncture modulation of cardiopulmonary bradycardia reflex.Am J Physiol Regul Integr Comp Physiol. 2014 Dec 1;307(11):R1313-23. doi: 10.1152/ajpregu.00300.2014. Epub 2014 Sep 17. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25231352 Free PMC article.
-
Modulation of cardiopulmonary depressor reflex in nucleus ambiguus by electroacupuncture: roles of opioids and γ-aminobutyric acid.Am J Physiol Regul Integr Comp Physiol. 2012 Apr;302(7):R833-44. doi: 10.1152/ajpregu.00440.2011. Epub 2011 Dec 28. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22204951 Free PMC article.
-
Modulation of Neurally Mediated Vasodepression and Bradycardia by Electroacupuncture through Opioids in Nucleus Tractus Solitarius.Sci Rep. 2018 Jan 30;8(1):1900. doi: 10.1038/s41598-018-19672-9. Sci Rep. 2018. PMID: 29382866 Free PMC article.
-
The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes.Ann N Y Acad Sci. 2001 Jun;940:48-58. Ann N Y Acad Sci. 2001. PMID: 11458703 Review.
-
Acupuncture's Cardiovascular Actions: A Mechanistic Perspective.Med Acupunct. 2013 Apr;25(2):101-113. doi: 10.1089/acu.2013.0960. Med Acupunct. 2013. PMID: 24761168 Free PMC article. Review.
Cited by
-
Key targets of signal transduction neural mechanisms in acupuncture treatment of cardiovascular diseases: Hypothalamus and autonomic nervous system.Heliyon. 2024 Sep 20;10(19):e38197. doi: 10.1016/j.heliyon.2024.e38197. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386880 Free PMC article. Review.
-
Does electroacupuncture reducing heart rate rebalance autonomic nervous system?J Neurophysiol. 2024 May 1;131(5):945-947. doi: 10.1152/jn.00132.2024. Epub 2024 Apr 24. J Neurophysiol. 2024. PMID: 38656173 Free PMC article. No abstract available.
-
Modulating activity of PVN neurons prevents atrial fibrillation induced circulation dysfunction by electroacupuncture at BL15.Chin Med. 2023 Oct 17;18(1):135. doi: 10.1186/s13020-023-00841-6. Chin Med. 2023. PMID: 37848944 Free PMC article.
-
Neurophysiological Basis of Electroacupuncture Stimulation in the Treatment of Cardiovascular-Related Diseases: Vagal Interoceptive Loops.Brain Behav. 2024 Oct;14(10):e70076. doi: 10.1002/brb3.70076. Brain Behav. 2024. PMID: 39344397 Free PMC article. Review.
References
-
- Aviado D. M., Guevara A. D. (2001). The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann. N. Y. Acad. Sci. 940 48–58. - PubMed
-
- Barman S., Phillips S., Gebber G. (2005). Medullary lateral tegmental field mediates the cardiovascular but not respiratory components of the Bezold-Jarisch reflex in the cat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289 R1693–R1702. - PubMed
-
- Chao D. M., Shen L. L., Tjen-A-Looi S. C., Pitsillides K. F., Li P., Longhurst J. C. (1999). Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am. J. Physiol. 276 H2127–H2134. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

