Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19
- PMID: 35959109
- PMCID: PMC9356643
- DOI: 10.1016/j.jcvp.2022.100101
Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19
Abstract
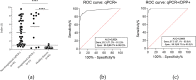
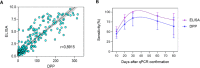
There is a massive demand to identify alternative methods to detect new cases of COVID-19 as well as to investigate the epidemiology of the disease. In many countries, importation of commercial kits poses a significant impact on their testing capacity and increases the costs for the public health system. We have developed an ELISA to detect IgG antibodies against SARS-CoV-2 using a recombinant viral nucleocapsid (rN) protein expressed in E. coli. Using a total of 894 clinical samples we showed that the rN-ELISA was able to detect IgG antibodies against SARS-CoV-2 with high sensitivity (97.5%) and specificity (96.3%) when compared to a commercial antibody test. After three external validation studies, we showed that the test accuracy was higher than 90%. The rN-ELISA IgG kit constitutes a convenient and specific method for the large-scale determination of SARS-CoV-2 antibodies in human sera with high reliability.
Keywords: COVID-19; Diagnosis; ELISA; Prototyping; SARS-CoV-2; Serological assay.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest. Funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Sabino E.C., Buss L.F., Carvalho M.P.S., Prete C.A., Crispim M.A.E., Fraiji N.A., Pereira R.H.M., Parag K.V, da Silva Peixoto P., Kraemer M.U.G., Oikawa M.K., Salomon T., Cucunuba Z.M., Castro M.C., de Souza Santos A.A., Nascimento V.H., Pereira H.S., Ferguson N.M., Pybus O.G., Kucharski A., Busch M.P., Dye C., Faria N.R. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397:452–455. doi: 10.1016/s0140-6736(21)00183-5. - DOI - PMC - PubMed
-
- Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, Coronavirus COVID-19 global cases, (2021). https://coronavirus.jhu.edu/map.html (Accessed July 07, 2022).
-
- Traugott M., Aberle S.W., Aberle J.H., Griebler H., Karolyi M., Pawelka E., Puchhammer-Stöckl E., Zoufaly A., Weseslindtner L. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid tests. J. Infect. Dis. 2020;222:362–366. doi: 10.1093/infdis/jiaa305. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
