Preferentially Expressed Antigen in Melanoma Immunohistochemistry Labeling in Uveal Melanomas
- PMID: 35959159
- PMCID: PMC9218614
- DOI: 10.1159/000524051
Preferentially Expressed Antigen in Melanoma Immunohistochemistry Labeling in Uveal Melanomas
Abstract
Introduction: Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, and despite treatment of the primary tumor, approximately 15%-50% of patients will develop metastatic disease. Based on gene expression profiling (GEPs), UM can be categorized as Class 1A (low metastatic risk), Class 1B (intermediate metastatic risk), or Class 2 (high metastatic risk). PReferentially expressed Antigen in MElanoma (PRAME) status is an independent prognostic UM biomarker and a potential target for immunotherapy in metastatic UM. PRAME expression status can be detected in tumors using reverse-transcription polymerase chain reaction (RT-PCR). More recently, immunohistochemistry (IHC) has been developed to detect PRAME protein expression. Here, we employed both techniques to evaluate PRAME expression in 18 UM enucleations.
Methods: Tumor material from the 18 UM patients who underwent enucleation was collected by fine-needle aspiration before or during enucleation and sent for GEP and PRAME analysis by RT-PCR. Histologic sections from these patients were stained with an anti-PRAME monoclonal antibody. We collected patient demographics and tumor characteristics and included this with our analysis of GEP class, PRAME status by RT-PCR, and PRAME status by IHC. PRAME IHC and RT-PCR results were compared.
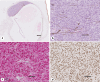
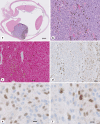
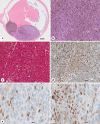
Results: Twelve males (12/18) and 6 females (6/18) with an average age of 60.6 years underwent enucleation for UM. TNM staging of the UM diagnosed Stage I in 2 patients (2/18), Stage II in 7 patients (7/18), Stage III in 8 patients (8/18), and Stage IV in 1 (1/18). GEP was Class 1A in 6 tumors (6/18), Class 1B in 6 tumors (6/18), and Class 2 in 6 tumors (6/18). PRAME IHC showed diffusely positive labeling of all UM cells in 2/18 enucleations; negative IHC labeling of UM cells in 9/18 enucleations; and IHC labeling of subsets of UM cells in 7/18 enucleations. Eleven of the 17 UMs tested for PRAME by both RT-PCR and IHC had consistent PRAME results. In the remaining 6/17 cases tested by both modalities, PRAME results were discordant between RT-PCR and IHC.
Conclusions: We find that PRAME IHC distinguishes PRAME-positive and PRAME-negative UM tumor cells. Interestingly, IHC reveals focal PRAME expression in subsets of tumor cells consistent with tumor heterogeneity. PRAME RT-PCR and IHC provide concordant results in most of our cases. We suggest that discordance in PRAME results could arise from spatial or temporal variation in PRAME expression between tumor cells. Further studies are required to determine the prognostic implications of PRAME IHC in UM.
Keywords: Immunohistochemistry; Metastasis; Preferentially expressed antigen in melanoma; Prognosis; Reverse-transcription polymerase chain reaction; Uveal melanoma.
Copyright © 2022 by S. Karger AG, Basel.
Conflict of interest statement
All authors: no reported conflicts.
Figures

Similar articles
-
Relationship between clinical features, GEP class, and PRAME expression in uveal melanoma.Graefes Arch Clin Exp Ophthalmol. 2019 Jul;257(7):1541-1545. doi: 10.1007/s00417-019-04335-w. Epub 2019 May 7. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31065847
-
COMPREHENSIVE MOLECULAR PROFILING OF UVEAL MELANOMA EVALUATED WITH GENE EXPRESSION PROFILING, PREFERENTIALLY EXPRESSED ANTIGEN IN MELANOMA EXPRESSION, AND NEXT-GENERATION SEQUENCING.Retina. 2024 Sep 1;44(9):1580-1589. doi: 10.1097/IAE.0000000000004153. Retina. 2024. PMID: 39167579
-
PRAME as a Potential Target for Immunotherapy in Metastatic Uveal Melanoma.JAMA Ophthalmol. 2017 Jun 1;135(6):541-549. doi: 10.1001/jamaophthalmol.2017.0729. JAMA Ophthalmol. 2017. PMID: 28448663 Free PMC article.
-
New concepts in the molecular understanding of uveal melanoma.Curr Opin Ophthalmol. 2017 May;28(3):219-227. doi: 10.1097/ICU.0000000000000366. Curr Opin Ophthalmol. 2017. PMID: 28257297 Review.
-
Recent advancements in the management of retinoblastoma and uveal melanoma.Fac Rev. 2021 May 28;10:51. doi: 10.12703/r/10-51. eCollection 2021. Fac Rev. 2021. PMID: 34195690 Free PMC article. Review.
Cited by
-
Stereotactic radiotherapy for uveal melanoma: A case report.Mol Clin Oncol. 2024 Jan 30;20(3):23. doi: 10.3892/mco.2024.2721. eCollection 2024 Mar. Mol Clin Oncol. 2024. PMID: 38357672 Free PMC article.
-
Enhanced diagnostic potential of CSPG4 in melanoma and nevi: a comparative study with PRAME, CDC7 and Ki67.J Pathol. 2025 Sep;267(1):69-78. doi: 10.1002/path.6450. Epub 2025 Jul 23. J Pathol. 2025. PMID: 40700516 Free PMC article.
-
Standardized Computer-Assisted Analysis of PRAME Immunoreactivity in Dysplastic Nevi and Superficial Spreading Melanomas.Int J Mol Sci. 2023 Mar 28;24(7):6388. doi: 10.3390/ijms24076388. Int J Mol Sci. 2023. PMID: 37047361 Free PMC article.
-
Immunohistochemistry for PRAME in Dermatopathology.Am J Dermatopathol. 2023 Nov 1;45(11):733-747. doi: 10.1097/DAD.0000000000002440. Am J Dermatopathol. 2023. PMID: 37856737 Free PMC article.
References
-
- Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern MH, et al. Uveal melanoma. Nat Rev Dis Primers. 2020 Apr 9;6((1)):24. - PubMed
-
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep;118((9)):1881–5. - PubMed
-
- Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012 Jul;32((7)):1363–72. - PubMed
-
- Manschot WA, Van Peperzeel HA. Uveal melanoma: location, size, cell type, and enucleation as risk factors in metastasis. Hum Pathol. 1982 Dec;13((12)):1147–8. - PubMed
-
- Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003 Nov;44((11)):4651–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

