Single channel approach for filtering electroencephalographic signals strongly contaminated with facial electromyography
- PMID: 35959164
- PMCID: PMC9361713
- DOI: 10.3389/fncom.2022.822987
Single channel approach for filtering electroencephalographic signals strongly contaminated with facial electromyography
Abstract
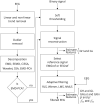
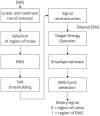
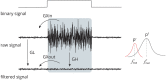
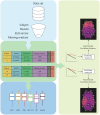
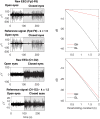
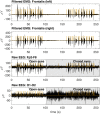
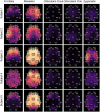
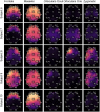
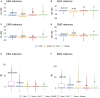
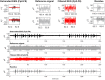
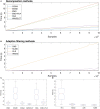
Eliminating facial electromyographic (EMG) signal from the electroencephalogram (EEG) is crucial for the accuracy of applications such as brain computer interfaces (BCIs) and brain functionality measurement. Facial electromyography typically corrupts the electroencephalogram. Although it is possible to find in the literature a number of multi-channel approaches for filtering corrupted EEG, studies employing single-channel approaches are scarce. In this context, this study proposed a single-channel method for attenuating facial EMG noise from contaminated EEG. The architecture of the method allows for the evaluation and incorporation of multiple decomposition and adaptive filtering techniques. The decomposition method was responsible for generating EEG or EMG reference signals for the adaptive filtering stage. In this study, the decomposition techniques CiSSA, EMD, EEMD, EMD-PCA, SSA, and Wavelet were evaluated. The adaptive filtering methods RLS, Wiener, LMS, and NLMS were investigated. A time and frequency domain set of features were estimated from experimental signals to evaluate the performance of the single channel method. This set of characteristics permitted the characterization of the contamination of distinct facial muscles, namely Masseter, Frontalis, Zygomatic, Orbicularis Oris, and Orbicularis Oculi. Data were collected from ten healthy subjects executing an experimental protocol that introduced the necessary variability to evaluate the filtering performance. The largest level of contamination was produced by the Masseter muscle, as determined by statistical analysis of the set of features and visualization of topological maps. Regarding the decomposition method, the SSA method allowed for the generation of more suitable reference signals, whereas the RLS and NLMS methods were more suitable when the reference signal was derived from the EEG. In addition, the LMS and RLS methods were more appropriate when the reference signal was the EMG. This study has a number of practical implications, including the use of filtering techniques to reduce EEG contamination caused by the activation of facial muscles required by distinct types of studies. All the developed code, including examples, is available to facilitate a more accurate reproduction and improvement of the results of this study.
Keywords: EEG; EMG; adaptive filtering; facial electromyography; signal decomposition.
Copyright © 2022 Queiroz, da Silva, Walter, Peres, Luiz, Costa, de Faria, Pereira, Vieira, Cabral and Andrade.
Figures





Similar articles
-
Emotion recognition from single-channel EEG signals using a two-stage correlation and instantaneous frequency-based filtering method.Comput Methods Programs Biomed. 2019 May;173:157-165. doi: 10.1016/j.cmpb.2019.03.015. Epub 2019 Mar 22. Comput Methods Programs Biomed. 2019. PMID: 31046991
-
Improving EEG Muscle Artifact Removal With an EMG Array.IEEE Trans Instrum Meas. 2020 Mar;69(3):815-824. doi: 10.1109/tim.2019.2906967. Epub 2019 May 1. IEEE Trans Instrum Meas. 2020. PMID: 32205896 Free PMC article.
-
EMG Signal Filtering Based on Variational Mode Decomposition and Sub-Band Thresholding.IEEE J Biomed Health Inform. 2021 Jan;25(1):47-58. doi: 10.1109/JBHI.2020.2987528. Epub 2021 Jan 5. IEEE J Biomed Health Inform. 2021. PMID: 32305948
-
Reducing Noise, Artifacts and Interference in Single-Channel EMG Signals: A Review.Sensors (Basel). 2023 Mar 8;23(6):2927. doi: 10.3390/s23062927. Sensors (Basel). 2023. PMID: 36991639 Free PMC article. Review.
-
Digital Filtering and Signal Decomposition: A Priori and Adaptive Approaches in Body Area Sensing.Biomed Eng Comput Biol. 2023 Apr 15;14:11795972231166236. doi: 10.1177/11795972231166236. eCollection 2023. Biomed Eng Comput Biol. 2023. PMID: 37077619 Free PMC article. Review.
References
-
- Abo-Zahhad M., Ahmed S. M., Abbas S. N. (2015). A new EEG acquisition protocol for biometric identification using eye blinking signals. Int. J. Intell. Syst. Appl. 7, 48–54. 10.5815/ijisa.2015.06.05 - DOI
-
- Alam M. E., Samanta B. (2017). Performance evaluation of empirical mode decomposition for EEG artifact removal, in Volume 4B: Dynamics, Vibration, and Control (Tampa, Florida: American Society of Mechanical Engineers; ), 1–8. 10.1115/IMECE2017-71647 - DOI
-
- Albera L., Kachenoura A., Comon P., Karfoul A., Wendling F., Senhadji L., et al. . (2012). ICA-based EEG denoising: a comparative analysis of fifteen methods. Bull. Pol. Acad. Sci. 60, 407–418. 10.2478/v10175-012-0052-3 - DOI
-
- Andrade A. O., Nasuto S., Kyberd P., Sweeney-Reed C. M., Kanijn F. V. (2006). EMG signal filtering based on empirical mode decomposition. Biomed. Signal Process. Control 1, 44–55. 10.1016/j.bspc.2006.03.003 - DOI
-
- Andrade A. O., Pereira A. A., Pinheiro C. G., Jr, Kyberd P. J. (2013). Mouse emulation based on facial electromyogram. Biomed. Signal Process. Control 8, 142–152. 10.1016/j.bspc.2012.09.001 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

