Patterned Hippocampal Stimulation Facilitates Memory in Patients With a History of Head Impact and/or Brain Injury
- PMID: 35959242
- PMCID: PMC9358788
- DOI: 10.3389/fnhum.2022.933401
Patterned Hippocampal Stimulation Facilitates Memory in Patients With a History of Head Impact and/or Brain Injury
Erratum in
-
Corrigendum: Patterned hippocampal stimulation facilitates memory in patients with a history of head impact and/or brain injury.Front Hum Neurosci. 2022 Oct 6;16:1039221. doi: 10.3389/fnhum.2022.1039221. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36277045 Free PMC article.
Abstract
Rationale: Deep brain stimulation (DBS) of the hippocampus is proposed for enhancement of memory impaired by injury or disease. Many pre-clinical DBS paradigms can be addressed in epilepsy patients undergoing intracranial monitoring for seizure localization, since they already have electrodes implanted in brain areas of interest. Even though epilepsy is usually not a memory disorder targeted by DBS, the studies can nevertheless model other memory-impacting disorders, such as Traumatic Brain Injury (TBI).
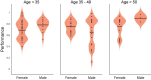
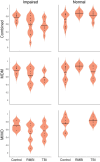
Methods: Human patients undergoing Phase II invasive monitoring for intractable epilepsy were implanted with depth electrodes capable of recording neurophysiological signals. Subjects performed a delayed-match-to-sample (DMS) memory task while hippocampal ensembles from CA1 and CA3 cell layers were recorded to estimate a multi-input, multi-output (MIMO) model of CA3-to-CA1 neural encoding and a memory decoding model (MDM) to decode memory information from CA3 and CA1 neuronal signals. After model estimation, subjects again performed the DMS task while either MIMO-based or MDM-based patterned stimulation was delivered to CA1 electrode sites during the encoding phase of the DMS trials. Each subject was sorted (post hoc) by prior experience of repeated and/or mild-to-moderate brain injury (RMBI), TBI, or no history (control) and scored for percentage successful delayed recognition (DR) recall on stimulated vs. non-stimulated DMS trials. The subject's medical history was unknown to the experimenters until after individual subject memory retention results were scored.
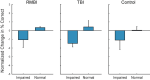
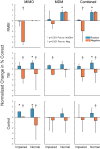
Results: When examined compared to control subjects, both TBI and RMBI subjects showed increased memory retention in response to both MIMO and MDM-based hippocampal stimulation. Furthermore, effects of stimulation were also greater in subjects who were evaluated as having pre-existing mild-to-moderate memory impairment.
Conclusion: These results show that hippocampal stimulation for memory facilitation was more beneficial for subjects who had previously suffered a brain injury (other than epilepsy), compared to control (epilepsy) subjects who had not suffered a brain injury. This study demonstrates that the epilepsy/intracranial recording model can be extended to test the ability of DBS to restore memory function in subjects who previously suffered a brain injury other than epilepsy, and support further investigation into the beneficial effect of DBS in TBI patients.
Keywords: deep brain stimulation; epilepsy; hippocampus; memory; memory decoding; memory encoding; non-linear dynamics; traumatic brain injury.
Copyright © 2022 Roeder, Riley, She, Dakos, Robinson, Moore, Couture, Laxton, Popli, Clary, Sam, Heck, Nune, Lee, Liu, Shaw, Gong, Marmarelis, Berger, Deadwyler, Song and Hampson.
Conflict of interest statement
RH discloses a current consulting and advisory relationship with Braingrade, Inc., a component of Engram (Holding), Inc., a Delaware C-Corporation. This relationship was not in effect at the time of the study. The remaining authors declare that the research was otherwise conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Berger T. W., Ahuja A., Courellis S. H., Deadwyler S. A., Erinjippurath G., Gerhardt G. A., et al. (2005). Restoring lost cognitive function. IEEE Eng. Med. Biol. Mag. 24 30–44. - PubMed
-
- Berger T. W., Glanzman D. L. (2005). Toward Replacement Parts for the Brain. Cambridge, MA: MIT Press.
LinkOut - more resources
Full Text Sources
Miscellaneous

