Nanoclay-Based Composite Films for Transdermal Drug Delivery: Development, Characterization, and in silico Modeling and Simulation
- PMID: 35959283
- PMCID: PMC9359522
- DOI: 10.2147/IJN.S367540
Nanoclay-Based Composite Films for Transdermal Drug Delivery: Development, Characterization, and in silico Modeling and Simulation
Abstract
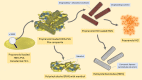
Purpose: Halloysite nanotubes (HNTs) are a versatile and highly investigated clay mineral due to their natural availability, low cost, strong mechanical strength, biocompatibility, and binding properties. The present work explores its role for retarding and controlling the drug release from the composite polymer matrix material.
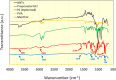
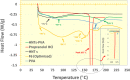
Methods: For this purpose, nanocomposite films comprising propranolol HCl and different concentrations of HNTs were formulated using the "solution casting method". The menthol in a concentration of 1% w/v was used as a permeation enhancer, and its effect on release and permeation was also determined. Quality characteristics of the nanocomposite were determined, and in vitro release and permeation studies were performed using the Franz diffusion system. The data was analyzed using various mathematical models and permeation parameters. Optimized formulation was also subjected to skin irritation test, FTIR, DSC, and SEM study. Systemic absorption and disposition of propranolol HCl from the nanocomposites were predicted using the GastroPlus TCAT® model.
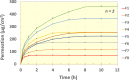
Results: The control in drug release rate was associated with the higher concentration of HNTs. F8 released 50% of propranolol within 8 hours (drug, HNTs ratio, 1:2). The optimized formulation (F6) with drug: HNTs (2:1), exhibited drug release 80% in 4 hours, with maximum flux of 145.812 µg/cm2hr. The optimized formulation was found to be a non-irritant for skin with a shelf life of 35.46 months (28-30 ℃). The in silico model predicted Cmax, Tmax, AUCt , and AUCinf as 32.113 ng/mL, 16.58 h, 942.34 ng/mL×h, and 1102.9 ng/mL×h, respectively.
Conclusion: The study demonstrated that HNTs could be effectively used as rate controlling agent in matrix type transdermal formulations.
Keywords: GastroPlus; controlled release; halloysite nanotube; nanocomposite; transdermal.
© 2022 Sikandar et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Formulation development, characterization, and mechanistic PBPK modeling of metoclopramide loaded halloysite nanotube (HNT) based drug-in-adhesive type transdermal drug delivery system.Sci Rep. 2024 Nov 18;14(1):28512. doi: 10.1038/s41598-024-80089-8. Sci Rep. 2024. PMID: 39558078 Free PMC article.
-
Formulation development and evaluation, in silico PBPK modeling and in vivo pharmacodynamic studies of clozapine matrix type transdermal patches.Sci Rep. 2025 Jan 7;15(1):1204. doi: 10.1038/s41598-024-81918-6. Sci Rep. 2025. PMID: 39774012 Free PMC article.
-
Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system.Drug Deliv Transl Res. 2017 Feb;7(1):66-76. doi: 10.1007/s13346-016-0334-7. Drug Deliv Transl Res. 2017. PMID: 27677866
-
Biomedical potential of clay nanotube formulations and their toxicity assessment.Expert Opin Drug Deliv. 2019 Nov;16(11):1169-1182. doi: 10.1080/17425247.2019.1665020. Epub 2019 Sep 12. Expert Opin Drug Deliv. 2019. PMID: 31486344 Review.
-
Halloysite nanotubes in analytical sciences and in drug delivery: A review.Mikrochim Acta. 2018 Jul 25;185(8):389. doi: 10.1007/s00604-018-2908-1. Mikrochim Acta. 2018. PMID: 30046919 Review.
Cited by
-
Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy.Gels. 2023 May 15;9(5):412. doi: 10.3390/gels9050412. Gels. 2023. PMID: 37233003 Free PMC article.
-
RSM and AI based machine learning for quality by design development of rivaroxaban push-pull osmotic tablets and its PBPK modeling.Sci Rep. 2025 Mar 7;15(1):7922. doi: 10.1038/s41598-025-91601-z. Sci Rep. 2025. PMID: 40050302 Free PMC article.
-
Formulation development, characterization, and mechanistic PBPK modeling of metoclopramide loaded halloysite nanotube (HNT) based drug-in-adhesive type transdermal drug delivery system.Sci Rep. 2024 Nov 18;14(1):28512. doi: 10.1038/s41598-024-80089-8. Sci Rep. 2024. PMID: 39558078 Free PMC article.
-
Formulation development and evaluation, in silico PBPK modeling and in vivo pharmacodynamic studies of clozapine matrix type transdermal patches.Sci Rep. 2025 Jan 7;15(1):1204. doi: 10.1038/s41598-024-81918-6. Sci Rep. 2025. PMID: 39774012 Free PMC article.
References
-
- Miller KJ. Transdermal product formulation development. In: Heather AE, Benson ACW, editors. Transdermal and Topical Drug Delivery - Principles and Practice. Firsted. Hoboken, New Jersey: John Wiley & Sons, Inc.; Vol. 1, 2012: 287.
-
- Ita K. Transdermal Drug Delivery - Concepts and Application. Vol. 1. UK: Elsevier; 2020.
-
- Chien YW. Novel Drug Delivery Systems. Seconded.Boca Raton, FL: CRC Press Taylor & Francis Group; Vol. 14, 1991.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

