Influences of Bifid Triple Viable Capsules Plus Cetirizine on Gut Microbiota and Immune Function in Children with Eczema
- PMID: 35959421
- PMCID: PMC9357560
- DOI: 10.2147/DDDT.S363702
Influences of Bifid Triple Viable Capsules Plus Cetirizine on Gut Microbiota and Immune Function in Children with Eczema
Abstract
Objective: Infantile eczema (IE), a common pediatric allergic skin disease caused by multiple inherent and external factors, is common in infants and young children, with skin lesions and itching as the main clinical manifestations. At present, its pathological mechanism has not been thoroughly clarified, but scholars believe that it is related to the joint action of various internal and external factors. This research aims to investigate the influences of bifid triple viable capsules plus cetirizine (CTZ) on gut microbiota (GMB) and immunity in children with eczema.
Methods: The complete clinical data of 162 cases of IE presented between July 2019 and July 2020 to the First Hospital of Shanxi Medical University were retrospectively analyzed. Children treated by CTZ alone were assigned to the control group (n = 81) and those by CTZ plus bifid triple viable capsules were included in the observation group (n = 81). Therapeutic efficacy, adverse reactions (ARs), disease recurrence, as well as changes in GMB, inflammatory factors (IFs) and immunoglobulins (Igs) were observed.
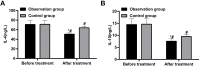
Results: The observation group was observed with a higher overall response rate and increased fecal Lactobacillus, Escherichia coli (E. coli) and Bifidobacterium counts after treatment versus the control group. After treatment, reduced IgG and IgM, as well as IFs, were found in both groups, with lower levels in the observation group. A lower incidence of ARs was determined in the observation group.
Conclusion: With high efficacy for the treatment of IE, bifid triple viable capsules plus CTZ can validly regulate the GMB of children, improve their immune function and clinical symptoms, and reduce the disease recurrence rate, which is worthy of clinical promotion.
Keywords: bifid triple viable capsules; cetirizine; eczema; gut microbiota; immune function.
© 2022 Su and Kang.
Conflict of interest statement
The authors declare no competing interests.
Figures
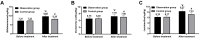
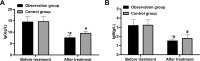
Similar articles
-
Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in mono-therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis.Clin Exp Allergy. 2004 Feb;34(2):259-67. doi: 10.1111/j.1365-2222.2004.01877.x. Clin Exp Allergy. 2004. PMID: 14987306 Clinical Trial.
-
Double-blind study of cetirizine in atopic eczema in children.Ann Allergy. 1994 Aug;73(2):117-22. Ann Allergy. 1994. PMID: 8067594 Clinical Trial.
-
Supplemental bifid triple viable capsule treatment improves inflammatory response and T cell frequency in ulcerative colitis patients.BMC Gastroenterol. 2021 Aug 4;21(1):314. doi: 10.1186/s12876-021-01887-2. BMC Gastroenterol. 2021. PMID: 34348654 Free PMC article.
-
Recent studies of cutaneous nociception in atopic and non-atopic subjects.J Dermatol. 1999 Feb;26(2):77-86. doi: 10.1111/j.1346-8138.1999.tb03516.x. J Dermatol. 1999. PMID: 10091477 Review.
-
[A meta-analysis of treatment of infantile diarrhea with bifid triple viable bacterial tablet].Zhonghua Er Ke Za Zhi. 2005 Jun;43(6):457-61. Zhonghua Er Ke Za Zhi. 2005. PMID: 16053735 Chinese.
Cited by
-
Effect of Different Doses of Vitamin D on the Intestinal Flora of Babies with Eczema: An Experimental Study.Life (Basel). 2022 Sep 9;12(9):1409. doi: 10.3390/life12091409. Life (Basel). 2022. PMID: 36143444 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

