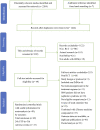
Efficacy and safety of 12 immunosuppressive agents for idiopathic membranous nephropathy in adults: A pairwise and network meta-analysis
- PMID: 35959430
- PMCID: PMC9358043
- DOI: 10.3389/fphar.2022.917532
Efficacy and safety of 12 immunosuppressive agents for idiopathic membranous nephropathy in adults: A pairwise and network meta-analysis
Abstract
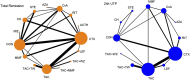
Background: Immunosuppressants have been applied in the remedy of idiopathic membranous nephropathy (IMN) extensively. Nevertheless, the efficacy and safety of immunosuppressants do not have final conclusion. Thus, a pairwise and network meta-analysis (NMA) was carried out to seek the most recommended therapeutic schedule for patients with IMN. Methods: Randomized controlled trials (RCTs) including cyclophosphamide (CTX), mycophenolate mofetil (MMF), tacrolimus-combined mycophenolate mofetil (TAC + MMF), cyclosporine (CsA), tacrolimus (TAC), leflunomide (LEF), chlorambucil (CH), azathioprine (AZA), adrenocorticotropic hormone (ACTH), non-immunosuppressive therapies (CON), steroids (STE), mizoribine (MZB), and rituximab (RIT) for patients with IMN were checked. Risk ratios (RRs) and standard mean difference (SMD) were reckoned to assess dichotomous variable quantities and continuous variable quantities, respectively. Total remission (TR) and 24-h urine total protein (24-h UTP) were compared using pairwise and NMA. Then interventions were ranked on the basis of the surface under the cumulative ranking curve (SUCRA). Results: Our study finally included 51 RCTs and 12 different immunosuppressants. Compared with the CON group, most regimens demonstrated better therapeutic effect in TR, with RR of 2.1 (95% CI) (1.5-2.9) for TAC, 1.9 (1.3-2.8) for RIT, 2.5 (1.2-5.2) for TAC + MMF, 1.9 (1.4-2.7) for CH, 1.8 (1.4-2.4) for CTX, 2.2 (1.0-4.7) for ACTH, 1.6 (1.2-2.1) for CsA, 1.6 (1.0-2.5) for LEF, and 1.6 (1.1-2.2) for MMF. In terms of 24-h UTP, TAC (SMD, -2.3 (95% CI -3.5 to -1.1)), CTX (SMD, -1.7 (95% CI -2.8 to -0.59)), RIT (SMD, -1.8 (95% CI -3.5 to -0.11)), CH (SMD, -2.4 (95% CI -4.3 to -0.49)), AZA (SMD, --4.2 (95% CI -7.7 to -0.68)), and CsA (SMD, -1.7 (95% CI -3 to -0.49)) were significantly superior than the CON group. As for adverse effects (AEs), infections, nausea, emesia, myelosuppression, and glucose intolerance were the collective adverse events for most immunosuppressants. Conclusion: This study indicates that TAC + MMF performed the best in terms of TR, and TAC shows the best effectiveness on 24-h UTP compared with other regimens. On the contrary, there seems to be little advantage on STE alone, LEF, AZA, and MZB in treating patients with IMN compared with CON. Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42021287013].
Keywords: adverse effects; idiopathic membranous nephropathy; immunosuppressant; network meta-analysis; therapies.
Copyright © 2022 Liu, Li, Huang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Comparative efficacy of 13 immunosuppressive agents for idiopathic membranous nephropathy in adults with nephrotic syndrome: a systematic review and network meta-analysis.BMJ Open. 2019 Sep 11;9(9):e030919. doi: 10.1136/bmjopen-2019-030919. BMJ Open. 2019. PMID: 31511292 Free PMC article.
-
Rituximab, tacrolimus, cyclophosphamide and cyclosporin in primary membranous nephropathy with nephrotic syndrome: comparison of safety profiles, effect on remission rate, 24-h urinary total protein, serum albumin, and serum creatinine levels using network meta-analysis.Int Urol Nephrol. 2025 May 8. doi: 10.1007/s11255-025-04549-4. Online ahead of print. Int Urol Nephrol. 2025. PMID: 40338506
-
Tacrolimus versus cyclophosphamide for patients with idiopathic membranous nephropathy and treated with steroids: a systematic review and meta-analysis of randomized controlled trials.Ren Fail. 2021 Dec;43(1):840-850. doi: 10.1080/0886022X.2021.1914655. Ren Fail. 2021. PMID: 34016023 Free PMC article.
-
Immunosuppressive treatment for nephrotic idiopathic membranous nephropathy: a meta-analysis based on Chinese adults.PLoS One. 2012;7(9):e44330. doi: 10.1371/journal.pone.0044330. Epub 2012 Sep 5. PLoS One. 2012. PMID: 22957065 Free PMC article.
-
Efficacy and safety of cyclosporine A in the treatment of idiopathic membranous nephropathy in an Asian population.Drug Des Devel Ther. 2019 Jul 11;13:2305-2330. doi: 10.2147/DDDT.S204974. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31371924 Free PMC article.
Cited by
-
Immunosuppressive Agent Options for Primary Nephrotic Syndrome: A Review of Network Meta-Analyses and Cost-Effectiveness Analysis.Medicina (Kaunas). 2023 Mar 17;59(3):601. doi: 10.3390/medicina59030601. Medicina (Kaunas). 2023. PMID: 36984602 Free PMC article. Review.
-
Effect of QingreHuoxue formula on Th17 cells and Tregs in mice with idiopathic membranous nephropathy.Am J Transl Res. 2024 Oct 15;16(10):5326-5336. doi: 10.62347/HJVG8103. eCollection 2024. Am J Transl Res. 2024. PMID: 39544812 Free PMC article.
-
Cyclophosphamide induced early remission and was superior to rituximab in idiopathic membranous nephropathy patients with high anti-PLA2R antibody levels.BMC Nephrol. 2023 Sep 22;24(1):280. doi: 10.1186/s12882-023-03307-x. BMC Nephrol. 2023. PMID: 37740193 Free PMC article.
-
Efficacy and safety of Tripterygium wilfordii multiglucoside for idiopathic membranous nephropathy: a systematic review with bayesian meta-analysis.Front Pharmacol. 2023 Aug 2;14:1183499. doi: 10.3389/fphar.2023.1183499. eCollection 2023. Front Pharmacol. 2023. PMID: 37608889 Free PMC article.
References
-
- Cameron J. S., Healy M. J., Adu D. (1990). The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. The MRC Glomerulonephritis Working Party. Q. J. Med. 74 (274), 133–156. 10.1093/oxfordjournals.qjmed.a068422 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

