Transient and Steady-State Properties of Drosophila Sensory Neurons Coding Noxious Cold Temperature
- PMID: 35959471
- PMCID: PMC9358291
- DOI: 10.3389/fncel.2022.831803
Transient and Steady-State Properties of Drosophila Sensory Neurons Coding Noxious Cold Temperature
Abstract
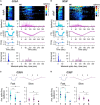
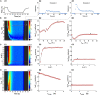
Coding noxious cold signals, such as the magnitude and rate of temperature change, play essential roles in the survival of organisms. We combined electrophysiological and computational neuroscience methods to investigate the neural dynamics of Drosophila larva cold-sensing Class III (CIII) neurons. In response to a fast temperature change (-2 to -6°C/s) from room temperature to noxious cold, the CIII neurons exhibited a pronounced peak of a spiking rate with subsequent relaxation to a steady-state spiking. The magnitude of the peak was higher for a higher rate of temperature decrease, while slow temperature decrease (-0.1°C/s) evoked no distinct peak of the spiking rate. The rate of the steady-state spiking depended on the magnitude of the final temperature and was higher at lower temperatures. For each neuron, we characterized this dependence by estimating the temperature of the half activation of the spiking rate by curve fitting neuron's spiking rate responses to a Boltzmann function. We found that neurons had a temperature of the half activation distributed over a wide temperature range. We also found that CIII neurons responded to decrease rather than increase in temperature. There was a significant difference in spiking activity between fast and slow returns from noxious cold to room temperature: The CIII neurons usually stopped activity abruptly in the case of the fast return and continued spiking for some time in the case of the slow return. We developed a biophysical model of CIII neurons using a generalized description of transient receptor potential (TRP) current kinetics with temperature-dependent activation and Ca2+-dependent inactivation. This model recapitulated the key features of the spiking rate responses found in experiments and suggested mechanisms explaining the transient and steady-state activity of the CIII neurons at different cold temperatures and rates of their decrease and increase. We conclude that CIII neurons encode at least three types of cold sensory information: the rate of temperature decrease by a peak of the firing rate, the magnitude of cold temperature by the rate of steady spiking activity, and direction of temperature change by spiking activity augmentation or suppression corresponding to temperature decrease and increase, respectively.
Keywords: Drosophila larvae; cold nociception; cold temperature magnitude; computational modeling; rate of temperature change; thermal sensation.
Copyright © 2022 Maksymchuk, Sakurai, Cox and Cymbalyuk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Cold-Temperature Coding with Bursting and Spiking Based on TRP Channel Dynamics in Drosophila Larva Sensory Neurons.Int J Mol Sci. 2023 Sep 27;24(19):14638. doi: 10.3390/ijms241914638. Int J Mol Sci. 2023. PMID: 37834085 Free PMC article.
-
Modality specific roles for metabotropic GABAergic signaling and calcium induced calcium release mechanisms in regulating cold nociception.Front Mol Neurosci. 2022 Sep 9;15:942548. doi: 10.3389/fnmol.2022.942548. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157080 Free PMC article.
-
The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila.Curr Biol. 2016 Dec 5;26(23):3116-3128. doi: 10.1016/j.cub.2016.09.038. Epub 2016 Nov 3. Curr Biol. 2016. PMID: 27818173 Free PMC article.
-
Time-Resolved Activation of Thermal TRP Channels by Fast Temperature Jumps.In: Zhu MX, editor. TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 14. In: Zhu MX, editor. TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 14. PMID: 22593957 Free Books & Documents. Review.
-
Ion channels involved in cold detection in mammals: TRP and non-TRP mechanisms.Biophys Rev. 2009 Dec;1(4):193-200. doi: 10.1007/s12551-009-0020-9. Epub 2009 Nov 10. Biophys Rev. 2009. PMID: 28510025 Free PMC article. Review.
Cited by
-
Neural substrates of cold nociception in Drosophila larva.bioRxiv [Preprint]. 2025 Jan 27:2023.07.31.551339. doi: 10.1101/2023.07.31.551339. bioRxiv. 2025. Update in: Elife. 2025 Jun 13;12:RP91582. doi: 10.7554/eLife.91582. PMID: 37577520 Free PMC article. Updated. Preprint.
-
Rapid threat assessment in the Drosophila thermosensory system.Nat Commun. 2023 Nov 3;14(1):7067. doi: 10.1038/s41467-023-42864-5. Nat Commun. 2023. PMID: 37923719 Free PMC article.
-
Neural substrates of cold nociception in Drosophila larva.Elife. 2025 Jun 13;12:RP91582. doi: 10.7554/eLife.91582. Elife. 2025. PMID: 40512662 Free PMC article.
-
Cold-Temperature Coding with Bursting and Spiking Based on TRP Channel Dynamics in Drosophila Larva Sensory Neurons.Int J Mol Sci. 2023 Sep 27;24(19):14638. doi: 10.3390/ijms241914638. Int J Mol Sci. 2023. PMID: 37834085 Free PMC article.
-
Modality specific roles for metabotropic GABAergic signaling and calcium induced calcium release mechanisms in regulating cold nociception.Front Mol Neurosci. 2022 Sep 9;15:942548. doi: 10.3389/fnmol.2022.942548. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157080 Free PMC article.
References
-
- Altner H., Loftus R. (1985). Ultrastructure and function of insect thermo- and hygroreceptors. Annu. Rev. Entomol. 30 273–295.
-
- Ameismeier F., Loftus R. (1988). Response characteristics of cold cell on the antenna of Locusta migratoria L. J. Comp. Physiol. A 163 507–516.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

