Characterizing single-molecule dynamics of viral RNA-dependent RNA polymerases with multiplexed magnetic tweezers
- PMID: 35959497
- PMCID: PMC9361327
- DOI: 10.1016/j.xpro.2022.101606
Characterizing single-molecule dynamics of viral RNA-dependent RNA polymerases with multiplexed magnetic tweezers
Abstract
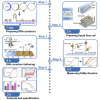
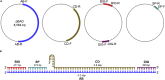
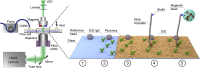
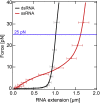
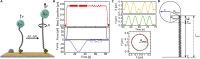
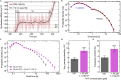
Multiplexed single-molecule magnetic tweezers (MT) have recently been employed to probe the RNA synthesis dynamics of RNA-dependent RNA polymerases (RdRp). Here, we present a protocol for simultaneously probing the RNA synthesis dynamics of hundreds of single polymerases with MT. We describe the preparation of a dsRNA construct for probing single RdRp kinetics. We then detail the measurement of RdRp RNA synthesis kinetics using MT. The protocol is suitable for high-throughput probing of RdRp-targeting antiviral compounds for mechanistic function and efficacy. For complete details on the use and execution of this protocol, please refer to Janissen et al. (2021).
Keywords: Biophysics; Microbiology; Molecular biology; Molecular/Chemical probes; Single-molecule assays.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Assessment of nucleotide/nucleoside analog intervention in primer-dependent viral RNA-dependent RNA polymerases.STAR Protoc. 2022 Jun 21;3(3):101468. doi: 10.1016/j.xpro.2022.101468. eCollection 2022 Sep 16. STAR Protoc. 2022. PMID: 35761985 Free PMC article.
-
Temperature controlled high-throughput magnetic tweezers show striking difference in activation energies of replicating viral RNA-dependent RNA polymerases.Nucleic Acids Res. 2020 Jun 4;48(10):5591-5602. doi: 10.1093/nar/gkaa233. Nucleic Acids Res. 2020. PMID: 32286652 Free PMC article.
-
Inhibition of SARS-CoV-2 polymerase by nucleotide analogs from a single-molecule perspective.Elife. 2021 Oct 7;10:e70968. doi: 10.7554/eLife.70968. Elife. 2021. PMID: 34617885 Free PMC article.
-
Antiviral therapeutics directed against RNA dependent RNA polymerases from positive-sense viruses.Mol Aspects Med. 2021 Oct;81:101005. doi: 10.1016/j.mam.2021.101005. Epub 2021 Jul 24. Mol Aspects Med. 2021. PMID: 34311994 Review.
-
State-of-the-Art Molecular Dynamics Simulation Studies of RNA-Dependent RNA Polymerase of SARS-CoV-2.Int J Mol Sci. 2022 Sep 8;23(18):10358. doi: 10.3390/ijms231810358. Int J Mol Sci. 2022. PMID: 36142270 Free PMC article. Review.
References
-
- Cnossen J.P., Dulin D., Dekker N.H. An optimized software framework for real-time, high-throughput tracking of spherical beads. Rev. Sci. Instrum. 2014;85:103712. - PubMed
-
- Dulin D., Vilfan I.D., Berghuis B.A., Hage S., Bamford D.H., Poranen M.M., Depken M., Dekker N.H. Elongation-competent pauses govern the fidelity of a viral RNA-dependent RNA polymerase. Cell Rep. 2015;10:983–992. - PubMed
-
- Dulin D., Berghuis B.A., Depken M., Dekker N.H. Untangling reaction pathways through modern approaches to high-throughput single-molecule force-spectroscopy experiments. Curr. Opin. Struct. Biol. 2015;34:116–122. - PubMed
-
- Fields B.N., Knipe D.M., Howley P.M. Lippincott-Raven Publishers; 1996. Fields Virology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

