Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury
- PMID: 35959683
- PMCID: PMC9426742
- DOI: 10.1161/CIRCRESAHA.121.320717
Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury
Abstract
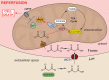
Background: Inhibiting SDH (succinate dehydrogenase), with the competitive inhibitor malonate, has shown promise in ameliorating ischemia/reperfusion injury. However, key for translation to the clinic is understanding the mechanism of malonate entry into cells to enable inhibition of SDH, its mitochondrial target, as malonate itself poorly permeates cellular membranes. The possibility of malonate selectively entering the at-risk heart tissue on reperfusion, however, remains unexplored.
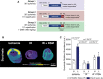
Methods: C57BL/6J mice, C2C12 and H9c2 myoblasts, and HeLa cells were used to elucidate the mechanism of selective malonate uptake into the ischemic heart upon reperfusion. Cells were treated with malonate while varying pH or together with transport inhibitors. Mouse hearts were either perfused ex vivo (Langendorff) or subjected to in vivo left anterior descending coronary artery ligation as models of ischemia/reperfusion injury. Succinate and malonate levels were assessed by liquid chromatography-tandem mass spectrometry LC-MS/MS, in vivo by mass spectrometry imaging, and infarct size by TTC (2,3,5-triphenyl-2H-tetrazolium chloride) staining.
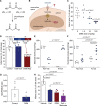
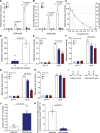
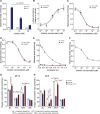
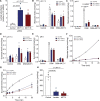
Results: Malonate was robustly protective against cardiac ischemia/reperfusion injury, but only if administered at reperfusion and not when infused before ischemia. The extent of malonate uptake into the heart was proportional to the duration of ischemia. Malonate entry into cardiomyocytes in vivo and in vitro was dramatically increased at the low pH (≈6.5) associated with ischemia. This increased uptake of malonate was blocked by selective inhibition of MCT1 (monocarboxylate transporter 1). Reperfusion of the ischemic heart region with malonate led to selective SDH inhibition in the at-risk region. Acid-formulation greatly enhances the cardioprotective potency of malonate.
Conclusions: Cardioprotection by malonate is dependent on its entry into cardiomyocytes. This is facilitated by the local decrease in pH that occurs during ischemia, leading to its selective uptake upon reperfusion into the at-risk tissue, via MCT1. Thus, malonate's preferential uptake in reperfused tissue means it is an at-risk tissue-selective drug that protects against cardiac ischemia/reperfusion injury.
Keywords: ischemia; mitochondria; myocardial infarction; reactive oxygen species; reperfusion.
Figures

Comment in
-
Targeted Mito- and Cardioprotection by Malonate.Circ Res. 2022 Sep 2;131(6):542-544. doi: 10.1161/CIRCRESAHA.122.321582. Epub 2022 Sep 1. Circ Res. 2022. PMID: 36048916 No abstract available.
References
-
- Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, et al. ; CARDIOPROTECTION COST Action (CA16225). Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086 - PubMed
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667 - PubMed
-
- Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, et al. . Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

