Effect of dentine site on resin and cement adaptation tested using X-ray and electron microscopy to evaluate bond durability and adhesive interfaces
- PMID: 35959863
- PMCID: PMC9805029
- DOI: 10.1111/eos.12890
Effect of dentine site on resin and cement adaptation tested using X-ray and electron microscopy to evaluate bond durability and adhesive interfaces
Abstract
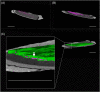
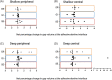
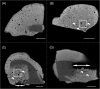
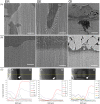
Glass ionomer (GI) cements and self-etch (SE) or universal adhesives after etching (ER) adapt variably with dentine. Dentine characteristics vary with depth (deep/shallow), location (central/peripheral), and microscopic site (intertubular/peritubular). To directly compare adhesion to dentine, non-destructive imaging and testing are required. Here, GI, ER, and SE adapted at different dentine depths, locations, and sites were investigated using micro-CT, xenon plasma focused ion beam scanning electron microscopy (Xe PFIB-SEM), and energy dispersive X-ray spectroscopy (EDS). Extracted molars were prepared to deep or shallow slices and treated with the three adhesives. Micro-CT was used to compare changes to air volume gaps, following thermocycling, and statistically analysed using a quantile regression model and Fisher's exact test. The three adhesives performed similarly across dentine depths and locations, yet no change or overall increases and decreases in gaps at all dentine depths and locations were measured. The Xe PFIB-SEM-milled dentine-adhesive interfaces facilitated high-resolution characterization, and element profiling revealed variations across the tooth-material interfaces. Dentine depth and location had no impact on adhesive durability, although microscopic differences were observed. Here we demonstrate how micro-CT and Xe PFIB-SEM can be used to compare variable dental materials without complex multi-stage specimen preparation to minimize artefacts.
Keywords: focused ion beam; humans; in vitro techniques; materials testing; surface properties.
© 2022 The Authors. European Journal of Oral Sciences published by John Wiley & Sons Ltd on behalf of Scandinavian Division of the International Association for Dental Research.
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- Chiba Y, Sunada N, Inoue N, Kawamoto R, Tonegawa M, Iwasa M, et al. Effect of operator variability on dentin bond strength of two‐step adhesive system. Adhes. Dent. 2007;25:239‐43.
-
- Perdigão J. Dentin bonding‐variables related to the clinical situation and the substrate treatment. Dent Mater 2010;26:e24‐37. - PubMed
-
- Tsujimoto A, Shimatani Y, Nojiri K, Barkmeier WW, Markham MD, Takamizawa T, et al. Influence of surface wetness on bonding effectiveness of universal adhesives in etch‐and‐rinse mode. Eur J Oral Sci 2019;127:162‐9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

