Enhanced development of functional human NK cells in NOD-scid-IL2rgnull mice expressing human IL15
- PMID: 35959876
- PMCID: PMC9383543
- DOI: 10.1096/fj.202200045R
Enhanced development of functional human NK cells in NOD-scid-IL2rgnull mice expressing human IL15
Abstract
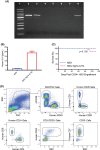
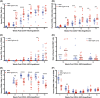
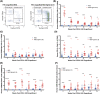
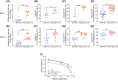
Human innate immunity plays a critical role in tumor surveillance and in immunoregulation within the tumor microenvironment. Natural killer (NK) cells are innate lymphoid cells that have opposing roles in the tumor microenvironment, including NK cell subsets that mediate tumor cell cytotoxicity and subsets with regulatory function that contribute to the tumor immune suppressive environment. The balance between effector and regulatory NK cell subsets has been studied extensively in murine models of cancer, but there is a paucity of models to study human NK cell function in tumorigenesis. Humanized mice are a powerful alternative to syngeneic mouse tumor models for the study of human immuno-oncology and have proven effective tools to test immunotherapies targeting T cells. However, human NK cell development and survival in humanized NOD-scid-IL2rgnull (NSG) mice are severely limited. To enhance NK cell development, we have developed NSG mice that constitutively expresses human Interleukin 15 (IL15), NSG-Tg(Hu-IL15). Following hematopoietic stem cell engraftment of NSG-Tg(Hu-IL15) mice, significantly higher levels of functional human CD56+ NK cells are detectable in blood and spleen, as compared to NSG mice. Hematopoietic stem cell (HSC)-engrafted NSG-Tg(Hu-IL15) mice also supported the development of human CD3+ T cells, CD20+ B cells, and CD33+ myeloid cells. Moreover, the growth kinetics of a patient-derived xenograft (PDX) melanoma were significantly delayed in HSC-engrafted NSG-Tg(Hu-IL15) mice as compared to HSC-engrafted NSG mice demonstrating that human NK cells have a key role in limiting the tumor growth. Together, these data demonstrate that HSC-engrafted NSG-Tg(Hu-IL15) mice support enhanced development of functional human NK cells, which limit the growth of PDX tumors.
Keywords: Hu-IL15; NK cells; NSG; humanized mice; transgenic.
© 2022 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures




Similar articles
-
Human Hematopoietic Stem Cell Engrafted IL-15 Transgenic NSG Mice Support Robust NK Cell Responses and Sustained HIV-1 Infection.Viruses. 2023 Jan 27;15(2):365. doi: 10.3390/v15020365. Viruses. 2023. PMID: 36851579 Free PMC article.
-
Humanized mouse model supports development, function, and tissue residency of human natural killer cells.Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):E9626-E9634. doi: 10.1073/pnas.1705301114. Epub 2017 Oct 25. Proc Natl Acad Sci U S A. 2017. PMID: 29078283 Free PMC article.
-
Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells.J Immunol. 2010 Sep 1;185(5):2710-20. doi: 10.4049/jimmunol.1000583. Epub 2010 Jul 28. J Immunol. 2010. PMID: 20668220
-
Current humanized mouse models for studying human immunology and HIV-1 immuno-pathogenesis.Sci China Life Sci. 2010 Feb;53(2):195-203. doi: 10.1007/s11427-010-0059-7. Epub 2010 Mar 7. Sci China Life Sci. 2010. PMID: 20596827 Free PMC article. Review.
-
Modeling normal and malignant human hematopoiesis in vivo through newborn NSG xenotransplantation.Int J Hematol. 2013 Dec;98(6):634-40. doi: 10.1007/s12185-013-1467-9. Epub 2013 Nov 21. Int J Hematol. 2013. PMID: 24258713 Review.
Cited by
-
ALK inhibitors suppress HCC and synergize with anti-PD-1 therapy and ABT-263 in preclinical models.iScience. 2024 Apr 23;27(5):109800. doi: 10.1016/j.isci.2024.109800. eCollection 2024 May 17. iScience. 2024. PMID: 38741708 Free PMC article.
-
HIV Persistence, Latency, and Cure Approaches: Where Are We Now?Viruses. 2024 Jul 19;16(7):1163. doi: 10.3390/v16071163. Viruses. 2024. PMID: 39066325 Free PMC article. Review.
-
Cytokine-induced memory-like NK cells combined with Tafasitamab demonstrate efficacy against B-cell acute lymphoblastic leukemia.Immunother Adv. 2025 Jul 16;5(1):ltaf025. doi: 10.1093/immadv/ltaf025. eCollection 2025. Immunother Adv. 2025. PMID: 40672109 Free PMC article.
-
Preclinical characterization of MTX-101: a novel bispecific CD8 Treg modulator that restores CD8 Treg functions to suppress pathogenic T cells in autoimmune diseases.Front Immunol. 2024 Nov 4;15:1452537. doi: 10.3389/fimmu.2024.1452537. eCollection 2024. Front Immunol. 2024. PMID: 39559361 Free PMC article.
-
From ex vivo to in vitro models: towards a novel approach to investigate the efficacy of immunotherapies on exhausted Vγ9Vδ2 T cells?Front Immunol. 2025 Apr 22;16:1556982. doi: 10.3389/fimmu.2025.1556982. eCollection 2025. Front Immunol. 2025. PMID: 40330479 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

