Two-pore channels: going with the flows
- PMID: 35959977
- PMCID: PMC9444070
- DOI: 10.1042/BST20220229
Two-pore channels: going with the flows
Abstract
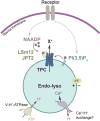
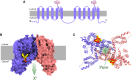
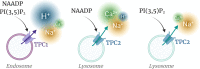
In recent years, our understanding of the structure, mechanisms and functions of the endo-lysosomal TPC (two-pore channel) family have grown apace. Gated by the second messengers, NAADP and PI(3,5)P2, TPCs are an integral part of fundamental signal-transduction pathways, but their array and plasticity of cation conductances (Na+, Ca2+, H+) allow them to variously signal electrically, osmotically or chemically. Their relative tissue- and organelle-selective distribution, together with agonist-selective ion permeabilities provides a rich palette from which extracellular stimuli can choose. TPCs are emerging as mediators of immunity, cancer, metabolism, viral infectivity and neurodegeneration as this short review attests.
Keywords: Ca2+; NAADP; TPC; lysosomes.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

